Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista de Osteoporosis y Metabolismo Mineral
versión On-line ISSN 2173-2345versión impresa ISSN 1889-836X
Rev Osteoporos Metab Miner vol.13 no.2 Madrid jun. 2021 Epub 16-Ago-2021
https://dx.doi.org/10.4321/s1889-836x2021000200004
ORIGINALS
Effect of vitamin D supplementation on aromatase inhibitor-related musculoskeletal side effects for breast cancer: B-ABLE cohort
1Hospital del Mar Institute for Medical Research (IMIM). Center for Biomedical Research Network on Frailty and Aging Healthy (CIBERFES). Barcelona (Spain)
2Cancer Research Program. Hospital del Mar Institute for Medical Research (IMIM). Barcelona (Spain)
3Department of Traumatology and Orthopedic Surgery. Alto Deba Hospital. Guipúzcoa (Spain)
4Department of Internal Medicine. Hospital del Mar. Autonomous University of Barcelona. Barcelona (Spain)
Objetive
To assess the effect of vitamin D supplementation on musculoskeletal complications related to aromatase inhibitor (AI) treatment in patients with breast cancer.
Material and methods
Prospective observational study of women undergoing AI treatment, recruited in the B-ABLE cohort. Patients with baseline serum 25 (OH) D (25-hydroxyvitamin D) levels <30 ng/ml received a 16,000 IU dose of oral calcifediol every 2 weeks. Arthralgia and bone loss related to AIs were assessed at 3 months and 1 year of followup, respectively. The association analyzes of vitamin D status at 3 months with musculoskeletal events were carried out using adjusted multivariate linear regression models. In addition, the association of incident pain, defined as patients without initial joint pain, but with a visual analog scale (VAS) >0 at 3 months, was evaluated using logistic regression.
Results
Vitamin D supplementation at the start of AI treatment decreased the risk of both incident arthralgia and its worsening. The effective threshold of 25 (OH) D in serum to reduce joint pain was established at 40 ng/ml. However, this threshold was not significantly related to bone changes at one year of follow-up. However, vitamin D levels were inversely correlated with lumbar spine bone loss (LS) (β=0.177% [95% CI: 0.014 to 0.340]).
Conclusions
Vitamin D supplementation aimed at achieving serum 25(OH)D levels of at least 40 ng/ml is protective for arthralgia. Vitamin D levels at three months could predict the risk of bone loss in LS at one year of AI treatment. Therefore, high doses of vitamin D are recommended in these patients, who are more prone to musculoskeletal conditions.
Key words aromatase inhibitors; vitamin D; osteoporosis; breast cancer; bone loss; arthralgia
INTRODUCTION
Survival for patients who suffer estrogen receptor positive (ER+) breast cancer has improved dramatically over the years due to the addition of adjuvant hormonal therapy, especially aromatase inhibitors (AI). Letrozole, anastrozole and exemestane are third generation AIs that massively reduce circulating estrogens in postmenopausal women. Although this effect is decisive for survival and the reduction of tumor relapse, it also leads to adverse events and quality of life problems, more prominently associated with the musculoskeletal system1.
Its use in women as adjunctive treatment for 2-5 years has been correlated with an increased risk of bone loss and fractures2,3. Furthermore, AI administration is associated with the appearance and/or increase of arthralgia –described as joint pain– with an estimated incidence of 55% in a previous study by our group4. The high rate of arthralgias is of particular concern, since it is reportedly the most frequent reason for interrupting treatment5,6. Although practical guidelines have been developed to prevent and manage IA-related bone loss7, effective treatment of arthralgia has yet to be addressed8.
Previous studies in the B-ABLE cohort, a clinical, prospective, cohort study of women diagnosed with early ER+ breast cancer, and candidates for aromatase inhibitor therapy, showed that low levels of 25-hydroxyvitamin D (25(OH)D) were associated with greater bone mass loss and worsening joint pain9-11. Similarly, IA-related arthralgia in the B-ABLE cohort was significantly reduced in those patients who achieved serum 25(OH)D concentrations ≥40 ng/ml11. Consequently, maintaining optimal 25(OH)D levels in the general population is strongly recommended to prevent not only bone loss but other nonskeletal disorders as well12. Therefore, assessment of serum 25(OH)D levels in breast cancer patients treated with AI could be important in preventing musculoskeletal disorders, as well as other issues that affect quality of life.
To further explore the association of vitamin D status with bone loss and arthralgia, the expanded B-ABLE cohort, comprised of 927 postmenopausal women diagnosed with RE+ breast cancer and treated with IA, was evaluated. This was intended to establish target 25 (OH) D threshold levels to prevent the appearance of arthralgias associated with AI.
MATERIAL AND METHODS
Study design and participants
From January 2006 to January 2019, data were collected from 927 Caucasian postmenopausal women who had been diagnosed with ER+ early breast cancer and who were candidates for AI treatment (letrozole, exemestane, or anastrozole). These women were recruited into the B-ABLE cohort –an unselected, prospective clinical cohort study– at Hospital del Mar (Barcelona, Spain) (ClinicalTrials.gov 2019 Identifier: NCT03811509).
Participants were recruited 6 weeks after surgery or 1 month after the last chemotherapy cycle or, alternatively, once menopause began after taking tamoxifen (TAM) for 2 to 3 years. Postmenopausal status was defined as patients aged >55 years with amenorrhea of >12 months, or those aged ≤55 years with luteinizing hormone levels >30 mIU/ml and/or follicle-stimulating hormone levels >40 mIU/ml. Exclusion criteria were: previous history of any metabolic bone disorder, alcoholism, rheumatoid arthritis, and concurrent or previous treatment with oral corticosteroids. Patients with vitamin D levels ≥30 ng/ml were also excluded, as they did not receive vitamin D supplements.
At the outset of the study, all patients' bone mineral density (BMD) was evaluated in the lumbar spine (L1-L4), the femoral neck (FN) and the total hip (TH). Those with a T-score <-2.5 at any site, or with a T-score ≤-2.0 at any site plus a major risk factor13, and/or previous fragility fractures, were treated with antiresorptive drugs, including weekly oral risedronate or alendronate, or denosumab every 6 months.
All participants with baseline serum levels of 25 (OH) D <30 ng/ml received a dose of 16,000 IU of oral calcifediol (Hidroferol® Faes Farma) every 2 weeks from the start of the study, in addition to calcium tablets and 25 (OH) vitamin D3 (1,000 mg and 800 IU daily, respectively) if your dietary calcium intake was less than 1,200 mg/day.
Variables
Visual analog scale
A visual analog scale (VAS) was used to record the intensity of self-reported joint pain at baseline (before starting AI treatment) and after 3 months of AI treatment.The score ranged from 0 (no pain) to 10 (maximum pain). The question associated with the VAS was the following ''please indicate the intensity of the pain you feel in your peripheral joints (knee, wrist, fingers/toes, elbow, shoulder, etc.), excluding the spine/back pain and pain in the operated area''11.
The administration of analgesics and anti-inflammatories was recorded and taken into account for the evaluation of pain.
Vitamin D levels
Vitamin D (25 (OH) D) levels were assessed at baseline and at 3-month follow-up in each study participant. Serum 25 (OH) D levels were obtained from peripheral blood using a competitive direct immunoluminometric assay with direct coated magnetic microparticles (coefficient of variation: <10%) (Elecsys Vitamin D total II, model 07028148190; Cobas e801 system, Roche Diagnostics GmbH, Mannheim, Germany).
Bone mineral density (BMD)
BMD measurements were made in the lumbar spine (LS), the neck of the femur (FN) and the total hip (TH) at the beginning and at 12 months of treatment with AI. BMD was measured with a DXA QDR 4500 SL® densitometer (Hologic, Waltham, Massachusetts, USA), according to the manufacturer's recommendations. In our unit, the in vivo coefficient of variation of this technique is 1.0% in LS, 1.60% in CT and 1.65% in CF.
Other variables
At the time of recruitment, data on clinical variables were recorded, such as: age, body mass index (BMI), age at menarche and menopause, number of children, total months of breastfeeding, spine x-ray and recent chemotherapy (women exposed to chemotherapy one month before recruitment), among others.
Statistical analysis
Descriptive data were presented using the mean or median depending on the nature of the variables. Differences between values at baseline and at 3 or 12 months were analyzed using the Wilcoxon paired samples test and the paired t test. Based on previous findings11, four vitamin D thresholds were defined according to the patients' vitamin D concentrations at three months of follow-up: ≥20 ng/ml, ≥30 ng/ml, ≥40 ng/ml and ≥50 ng/ml. ml. The association between absolute changes in VAS from baseline to 3 months and vitamin D thresholds was analyzed using a multivariate linear regression model. Furthermore, the association of incident pain, defined as patients without initial joint pain, but with a VAS >0 at 3 months, and vitamin D thresholds, was evaluated using logistic regression. Regression analyzes were adjusted for age, BMI, recent chemotherapy, previous use of tamoxifen, and current use of bone antiresorptives. The linearity, interaction and absence of multicollinearity of the independent variables were checked.
Finally, a subset of participants not exposed to antiresorptive treatments was selected to assess the association between relative changes in BMD at 12 months and vitamin D thresholds, or vitamin D levels at 3 months, using linear regression. adjusting for age, BMI, years since menopause, recent chemotherapy, and prior tamoxifen use. In addition, the linearity of the independent variables was verified.
Statistical analyzes were carried out using R for Windows version 3.3.3, using foreign, compareGroups, car, QuantPsyc and gam. All statistical tests with p<0.05 were considered significant.
Ethics approval
The study protocol followed the standards of the Declaration of Helsinki and was approved by the Parc de Salut Mar ethics committee (2016/6803/I). Written informed consent was obtained from each participant once they had read the study information sheet and all their doubts were clarified. The privacy rights of human subjects were always respected.
RESULTS
Participants
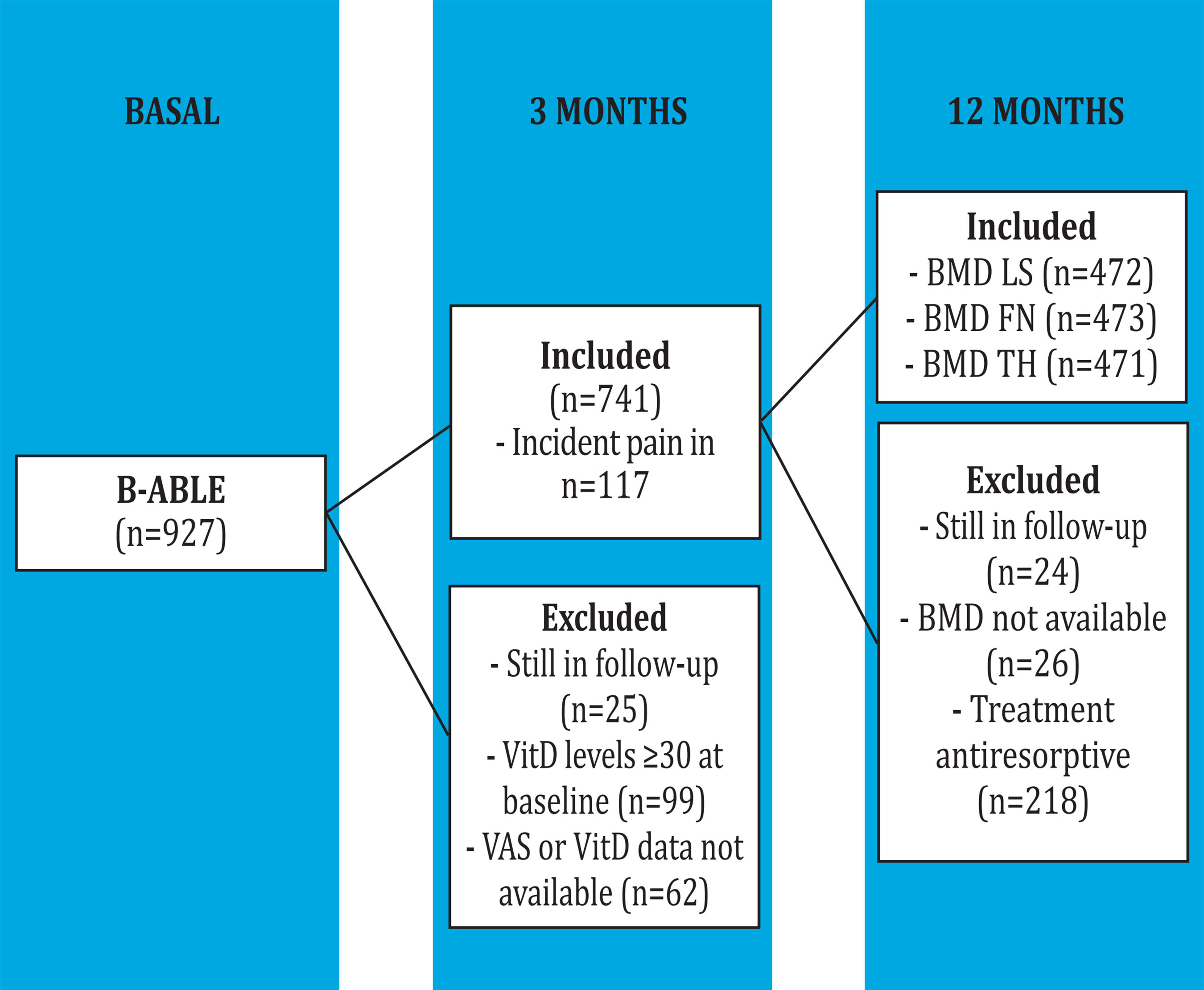
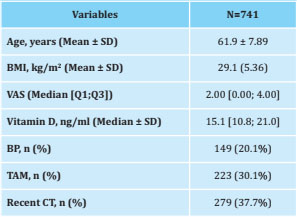
A total of 741 of the 927 patients recruited in the B-ABLE cohort were visited at the 3-month follow-up, had data available and had baseline serum 25 (OH) D levels below 30 ng/ml (Figure 1). and, therefore, they were eligible for the present study. The baseline characteristics of the selected patients are indicated in table 1.
AI-related arthralgia and vitamin D status at 3 months
At 3 months, the median [Q1;Q3] of the VAS increased from 2.00 [0.00;4.00] to 3.00 [0.00;5.00] (p<0.001), and the vitamin D increased from 15.10 [10.8;21.00] to 40.20 [30.90;52.50] (p<0.001). The change in VAS from baseline to 3 months was significantly associated with a vitamin D threshold ≥40 ng/ml (p<0.05) at 3 months of follow-up (Table 2), that is, an increase in VAS decreased 0.40 units in patients who reached a vitamin D threshold greater than 40 ng/ml with supplementation (Figure 2).
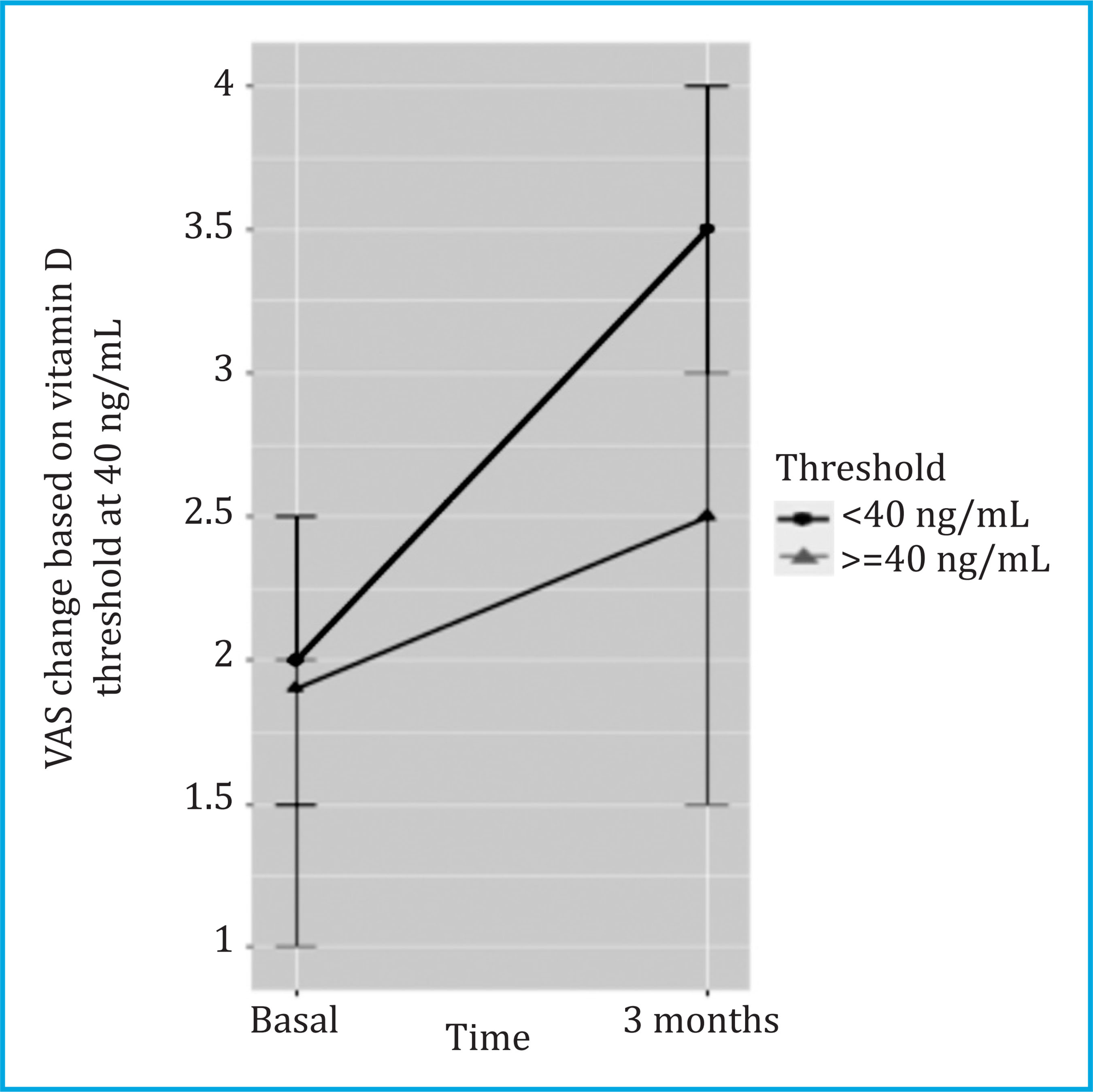
Figure 2. VAS changes are stratified by the vitamin D threshold of 40 ng/ml at 3 months, in all patients with baseline vitamin D levels ≤30 ng/ml. VAS values are reported as median [95% CI]
Table 2. Linear regression between the change in the VAS from baseline to 3 months and the vitamin D threshold at 3 months (in all patients n=741)
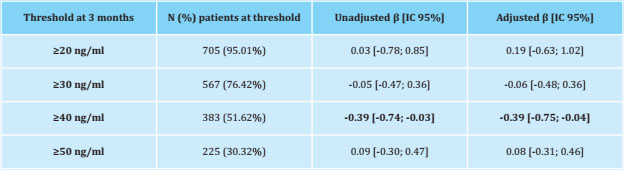
β: β-coefficient adjusted for age, BMI, recent chemotherapy, antiresorptive drugs and previous tamoxifen; CI: confidence interval. In bold: significant results (p<0.05).
Incident pain was assessed in a subset of 301 patients without initial pain. Of these, 117 (38.87%) developed joint pain at 3 months with a median VAS [Q1;Q3] of 3.50 [2.20;5.00]. The logistic regression between vitamin D thresholds and the appearance of joint pain at 3 months showed that those patients who achieved vitamin D le- vels ≥40 ng/ml were less likely to experience incident pain (p<0.05) (Table 3 and Figure 3).
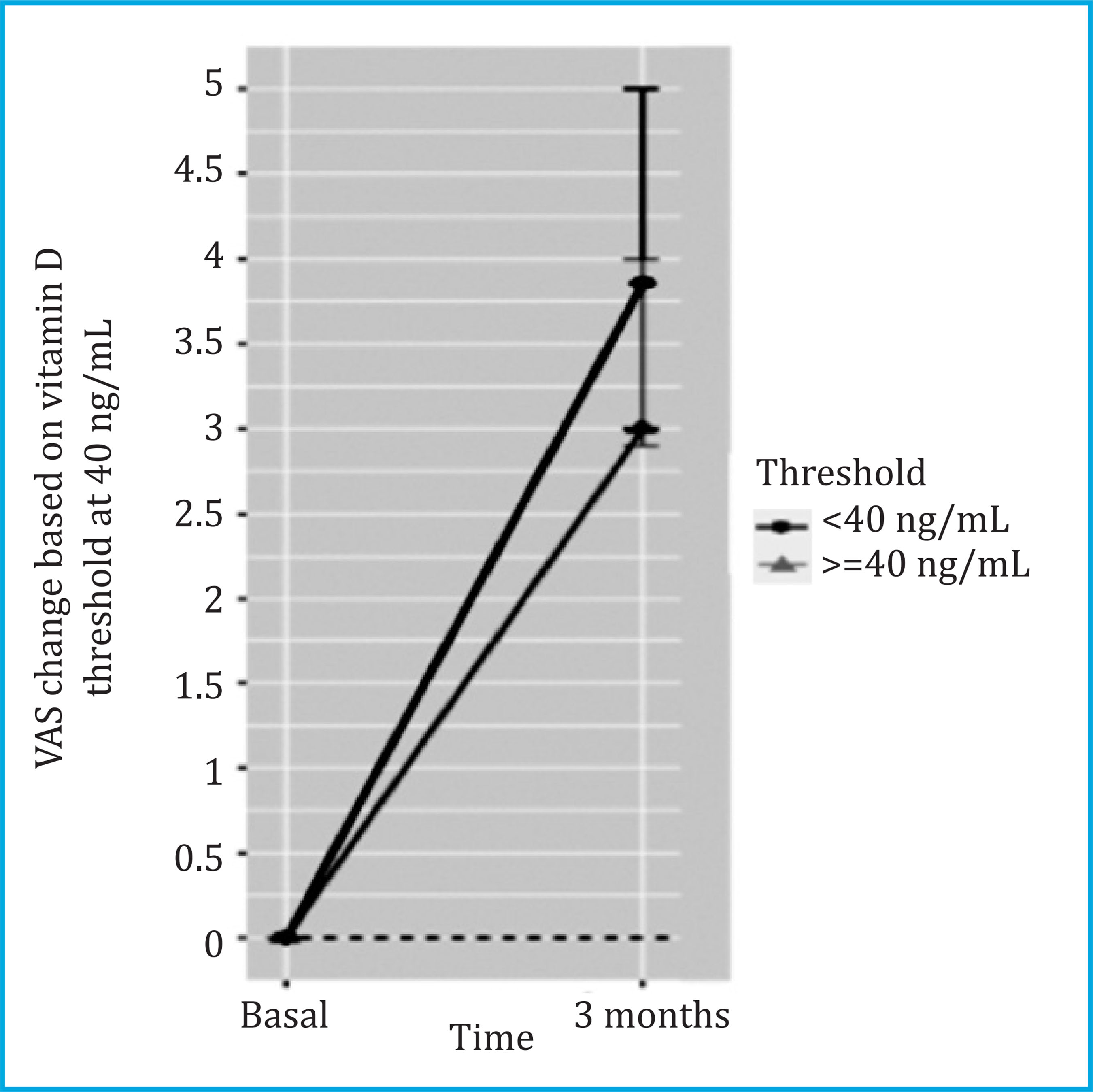
Figure 3. Changes in VAS in women with incident pain (n=117) and according to the vitamin D threshold of 40 ng/mL. VAS values are reported as median [95 CI)
Table 3. Logistic regression between incident pain and vitamin D threshold at 3 months (patients without initial pain n=301; of these, n=117 developed incident pain)
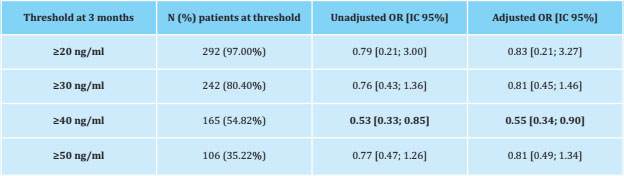
CI: confidence interval; OR: odds ratio, adjusted for: age, BMI, recent chemotherapy, antiresorptive drugs, and previous tamoxifen. In bold: significant results (p<0.05).
BMD and vitamin D status
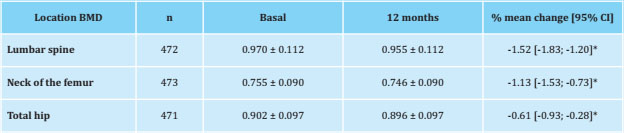
Data from 473 patients who were not exposed to any antiresorptive treatment and who had BMD data at 12 months of follow-up were analyzed. In these patients, the BMD of the LS, FN and TH decreased significantly after 12 months of treatment with AI (p<0.001) (Table 4).
No association was detected between any of the vitamin D thresholds analyzed (≥20 ng/ml, ≥30 ng/ml, ≥40 ng/ml, or ≥50 ng/ml) at 3 months and the relative changes in BMD of LS, FN and TH at 12 months. However, each 10 ng/ml increase in serum vitamin D at three months was associated with a lower loss of BMD in LS (unadjusted β = +0.194% [95% CI: 0.028 to 0.359] and adjusted β = +0.177% [95% CI: 0.014 to 0.340]; p<0.05). No significant associations were observed between vitamin D levels and BMD of FN and TH.
DISCUSSION
An observational, prospective, and real-life study of postmenopausal women treated with aromatase inhibitors included in the B-ABLE cohort was carried out. AI treatment in ER+ early breast cancer patients is strongly associated with musculoskeletal side effects. However, vitamin D supplementation early in AI appears to attenuate one of the main risk factors for treatment interruption: AI-related arthralgia. Our results suggest that AI-induced joint pain is vitamin D dependent, and that 40 ng/ml is the effective target threshold for serum 25 (OH) D levels to reduce the risk of both joint pain incidence and its worsening. However, this threshold is not significantly related to changes in BMD at one year of follow-up. However, vitamin D supplementation was inversely correlated with bone loss of CL, as each 10 ng/ml increase in serum 25 (OH) D at 3 months resulted in a reduction in blood pressure and 0.177% bone loss.
Vitamin D is known to play an important role in musculoskeletal tissues in addition to bone14, including muscle15, cartilage16, and synovium17. Previous studies carried out in women with ER+ early breast cancer receiving AI treatment, who also frequently present vitamin D deficiency18, provide evidence of the possible effects of vitamin D status on musculoskeletal health9,19. In our cohort study, the main musculoskeletal effect of vitamin D supplementation was found in AI-related arthralgia, consistent with a previous study by Prieto-Alhambra et al. in 201111. Similarly, another observational study showed that a high dose of vitamin D (50,000 IU weekly of vitamin D3 orally) improved arthralgia values in patients who achieved mean concentrations of 25 (OH) D higher than the mean of 66 ng/ml20. In our case, the threshold was defined as ≥40 ng/ml, which was reached after 3 months of vitamin D supplementation in approximately 50% of patients. Clinically, the fact of containing the increase in pain related to AI at 3 months helps to improve the patients' quality of life, as well as avoiding treatment discontinuity21.
Unlike pain, changes in BMD usually take longer to notice. Bone remodeling is a progressive process that results in long-term changes in BMD. Therapeutic interventions on BMD are evaluated annually in routine clinical practice. For this reason, in our study, BMD was assessed after 1 year of follow-up. Associations with vitamin D intake were only detected in the lumbar spine, which is not surprising given that bone remodeling is more active in this area and the pharmacological effects are more visible in trabecular bone compared to other skeletal locations with greater cortical content. We observed that increases in serum 25 (OH) D at 3 months were inversely correlated with AI-related bone loss at 1 year, therefore, this increase in 25 (OH) D could predict bone behavior at 1 year, but only visible in column. This coincides with a previous study by Prieto-Alhambra et al.22, although they found greater reductions in bone loss of 1.70%, in patients who achieved serum vitamin D levels ≥40 ng/ml.
This study has several limitations. First, this is not a randomized control trial, so the efficacy of high-dose vitamin D supplementation compared to a placebo group could not be assessed. Furthermore, compliance with vitamin D supplementation was not strictly controlled. This could explain the variability of 25 (OH) D levels between patients after 3 months of treatment. Finally, the current assumption that circulating 25(OH)D concentrations are a measure of vitamin D functional status may be incorrect. However, measuring 25(OH)D levels is the easiest and most reliable assessment of vitamin D status currently available.
Vitamin D supplementation administered to patients in specified doses increases levels from 15.10 [10.8;21.00] to 40.20 [30.90;52.50], thus reaching adequate levels of vitamin D at 3 months in most of the patients. The goal of therapy is to treat vitamin D insufficiency/deficiency rather than increase to supranormal concentrations, so we believe the risk of harm from administered doses is very low.
Our results suggest that optimal levels of vitamin D are associated with a reduced risk of joint pain related to AI treatment. A target threshold of 25OHD serum levels was set at 40 ng/ml to significantly reduce the increase in joint pain. It should be noted that this threshold is well above the goal of 20 ng/ml recommended by the 2010 Institute of Medicine (IOM) report23. Therefore, vitamin D supplements at the specified doses could be protective against arthralgia and AI-induced spinal bone loss. As a final observation, vitamin D supplements are inexpensive, safe, and easily accessible, making these drugs easy to use on a wide scale.
Funding: The authors declare that they have received search Training Projects (project numbers PI13/ the following financial support for the study: Center for 00444, PI16/00818) of the Carlos III Health Institute; Biomedical Research Network on Frailty and Healthy Sara Borrell grant from the Carlos III Health Institute, Aging (project number CB16/10/00245); Health Re-and the FEIOMM Research Grant 2019.
REFERENCES
1 Servitja S, Martos T, Rodriguez Sanz M, Garcia-Giralt N, Prieto-Alhambra D, Garrigos L, et al. Skeletal adverse effects with aromatase inhibitors in early breast cancer: evidence to date and clinical guidance. Ther Adv Med Oncol. 2015;7(5):291-296. [ Links ]
2 Rodríguez-Sanz M, Prieto-Alhambra D, Servitja S, Garcia-Giralt N, Garrigos L, Rodriguez-Morera J, et al. AI-related BMD variation in actual practice conditions: A prospective cohort study. Endocr Relat Cancer. 2016;23(4): 303-312. [ Links ]
3 Pineda-Moncusí M, Garcia-Giralt N, Diez-Perez A, Servitja S, Tusquets I, Prieto-Alhambra D, et al. Increased fracture risk in women treated with aromatase inhibitors versus tamoxifen: beneficial effect of bisphosphonates. J Bone Miner Res. 2020;35(2):291-297. [ Links ]
4 Garcia-Giralt N, Rodriguez-Sanz M, Prieto-Alhambra D, Servitja S, Torres-Del Pliego E, Balcells S, et al. Genetic determinants of aromatase inhibitor-related arthralgia: the B-ABLE cohort study. Breast Cancer Res Treat. 2013; 140(2):385-395. [ Links ]
5 Crew KD, Greenlee H, Capodice J, Raptis G, Brafman L, Fuentes D, et al. Prevalence of joint symptoms in postmenopausal women taking aromatase inhibitors for early-stage breast cancer. J Clin Oncol. 2007;25[25): 3877-3883. [ Links ]
6 Pineda-Moncusí M, Servitja S, Tus-quets I, Diez-Perez A, Rial A, Cos ML, et al. Assessment of early therapy discontinuation and health-related quality of life in breast cancer patients treated with aromatase inhibitors: BABLE cohort study. Breast Cancer Res Treat. 2019;177[1):53-60. [ Links ]
7 Reid DM, Doughty J, Eastell R, Heys SD, Howell A, McCloskey EV, et al. Guidance for the management of breast cancer treatment-induced bone loss: A consensus position statement from a UK Expert Group. Cancer Treat Rev. 2008;34(Suppl 1):S3-18. [ Links ]
8 Thorne C. Management of arthralgias associated with aromatase inhibitor therapy. Curr Oncol. 2007;14(Suppl 1):S11-19. [ Links ]
9 Nogues X, Servitja S, Peña MJ, Prieto-Alhambra D, Nadal R, Mellibovsky L, et al. Vitamin D deficiency and bone mineral density in postmenopausal women receiving aromatase inhibitors for early breast cancer. Maturitas. 2010;66(3):291-297. [ Links ]
10 Servitja S, Nogues X, Prieto-Alhambra D, Martinez-Garcia M, Garrigos L, Pena MJ, et al. Bone health in a prospective cohort of postmenopausal women receiving aromatase inhibitors for early breast cancer. Breast. 2012;21 (1):95-101. [ Links ]
11 Prieto-Alhambra D, Javaid MK, Servitja S, Arden NK, Martinez-Garcia M, Diez-Perez A, et al. Vitamin D threshold to prevent aromatase inhibitor-induced arthralgia: a prospective cohort study. Breast Cancer Res Treat. 2011;125(3): 869-878. [ Links ]
12 Giustina A, Adler RA, Binkley N, Bo-llerslev J, Bouillon R, Dawson-Hughes B, et al. Consensus statement from 2(nd) International Conference on Controversies in Vitamin D. Rev Endocr Metab Disord. 2020;21(1):89-116. [ Links ]
13 Rachner TD, Coleman R, Hadji P, Hof-bauer LC. Bone health during endocrine therapy for cancer. Lancet Diabetes Endocrinol. 2018;6(11):901-910. [ Links ]
14 Christakos S, Li S, DeLa Cruz J, Verlin-den L, Carmeliet G. Vitamin D and Bone. Handb Exp Pharmacol. 2020;262:47-63. [ Links ]
15 Garcia M, Seelaender M, Sotiropoulos A, Coletti D, Lancha AH Jr. Vitamin D, muscle recovery, sarcopenia, cachexia, and muscle atrophy. Nutrition. 2019;60:66-69. [ Links ]
16 Li S, Niu G, Dong XN, Liu Z, Song C, Leng H. Vitamin D inhibits activities of meta-lloproteinase-9/-13 in articular cartilage in vivo and in vitro. J Nutr Sci Vitaminol [Tokyo). 2019;65[2):107-112. [ Links ]
17 Sun HQ, Yan D, Wang QN, Meng HZ, Zhang YY, Yin LX, et al. 1,25-Dihydroxyvitamin D3 attenuates disease severity and induces synoviocyte apoptosis in a concentration-dependent manner in rats with adjuvant-induced arthritis by inactivating the NF-kappaB signaling pathway. J Bone Miner Metab. 2019;37(3):430-440. [ Links ]
18 Pineda-Moncusí M, Garcia-Perez MA, Rial A, Casamayor G, Cos ML, Servitja S, et al. Vitamin D levels in Mediterranean breast cancer patients compared with those in healthy women. Maturitas. 2018;116:83-88. [ Links ]
19 Rastelli A, Taylor M, Gao F, Armamento-Villareal R, Jamalabadi-Majidi S, Napoli N, et al. Vitamin D and aroma-tase inhibitor-induced musculoskeletal symptoms [AIMSS): a phase II, double-blind, placebo-controlled, randomized trial. Breast Cancer Res Treat. 2011;129[1):107-116. [ Links ]
20 Khan QJ, Reddy PS, Kimler BF, Sharma P, Baxa SE, O'Dea AP, et al. Effect of vitamin D supplementation on serum 25-hydroxy vitamin D levels, joint pain, and fatigue in women starting adjuvant letrozole treatment for breast cancer. Breast Cancer Res Treat. 2010; 119[1):111-118. [ Links ]
21 Kadakia KC, Snyder CF, Kidwell KM, Seewald MJ, Flockhart DA, Skaar TC, et al. Patient-reported outcomes and early discontinuation in aromatase inhibitor-treated postmenopausal women with early stagebreast cancer. Oncologist. 2016;21:539-556. [ Links ]
22 Prieto-Alhambra D, Servitja S, Javaid MK, Garrigos L, Arden NK, Cooper C, et al. Vitamin D threshold to prevent aro-matase inhibitor-related bone loss: the B-ABLE prospective cohort study. Breast Cancer Res Treat. 2012;133(3): 1159-1167. [ Links ]
23 Bouillon R, Van Schoor NM, Gielen E, Boonen S, Mathieu C, Vanderschueren D, et al. Optimal vitamin D status: a critical analysis on the basis of evidence-based medicine. J Clin Endocrinol Metab. 2013;98(8):E1283-1304. [ Links ]
Received: April 01, 2021; Accepted: May 22, 2021











 texto en
texto en