Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista de Osteoporosis y Metabolismo Mineral
versión On-line ISSN 2173-2345versión impresa ISSN 1889-836X
Rev Osteoporos Metab Miner vol.15 no.1 Madrid ene./mar. 2023 Epub 29-Mayo-2023
https://dx.doi.org/10.20960/revosteoporosmetabminer.00004
EDITORIAL
The role of parathyroid hormone related protein (PTHrP) in bone metabolism: from basic to clinical research
2Emeritus researcher. Instituto de Investigación Sanitaria-Fundación Jiménez Díaz. Madrid, Spain
Honorary Professor. Faculty of Pharmacy. Universidad Complutense de Madrid. Madrid, Spain
INTRODUCTION
Interest in parathyroid hormone related protein (PTHrP) emerged from cancer-associated hypercalcemia, the most common paraneoplastic syndrome affecting up to 20 % of patients with advanced cancer (1). Back in the 1980s it was reported that most patients with tumor hypercalcemia showed characteristics of pseudo-hyperparathyroidism, which prompted thinking of PTH or a similar factor secreted by the tumor as the culprit of this syndrome. It was at the end of this decade when three independent groups isolated and characterized the true causal factor, which, as it turned out to be, showed a structural similarity with PTH in its N-terminal end; hence the name it is known for, PTHrP (2-4). PTHrP elevated plasma levels have been detected in most patients with tumor hypercalcemia (5,6) in whom PTHrP induces an increase of bone resorption and tubular calcium resorption as the cause of hypercalcemia.
However, its characterization led to an unexpected result: PTHrP turned out to be a cytokine that is present in a wide variety of normal tissues, where it exerts auto/paracrine and/or intracrine actions; as a matter of fact, tumor hypercalcemia is one of the few situations in which PTHrP exerts endocrine actions due to tumor hypersecretion (7). Therefore, the discovery of PTHrP is a magnificent example of translational research in biomedicine: the clinical research of a paraneoplastic syndrome ended up with the discovery of a new cellular cytokine. As a matter of fact, as we will see below, PTHrP “has come back to clinical research” somehow, as today it is regarded as a new agent in the pharmacological armamentarium of bone-forming agents in osteoporosis.
PTHrP: A MULTIFUNCTIONAL CYTOKINE IN BONE METABOLISM
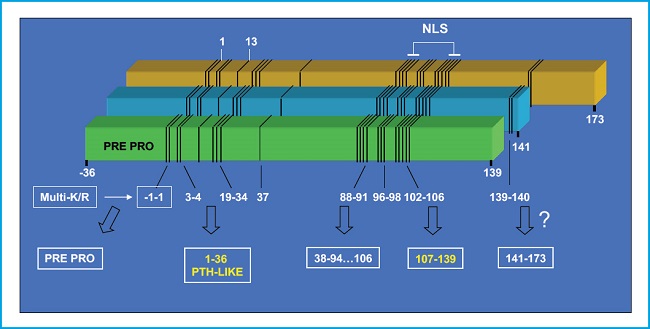
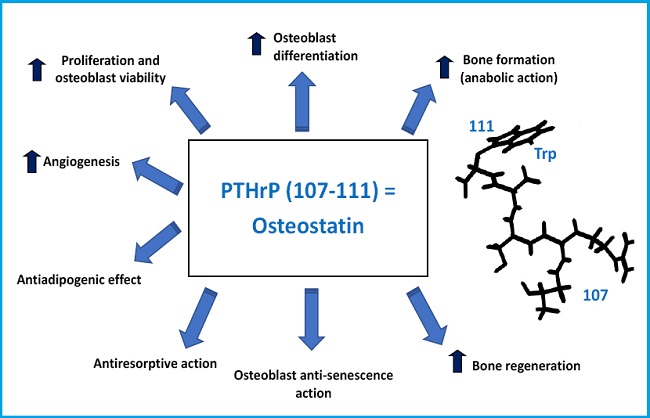
The PTHrP gene contains multiple exons and is located in the short arm of chromosome 12, in a position analogous to that of the PTH gene in chromosome 11, and they both share a common ancestral origin. By alternative mRNA processing, the PTHrP gene in humans results in 3 protein isoforms of 139, 141, and 173 amino acids. Its proteolytic breakdown creates several fragments with different bioactivity (7,8) (Fig. 1). Its N-terminal fragment contains structural similarities with PTH, in both 1-13 and 14-34 regions, which allows its interaction with the same PTH receptor type 1 (PTHR1) (9). The middle region contains a nuclear/nucleolus localization domain (NLS) with specific functional properties in several cellular types including osteoblasts (10). The C-terminal fragment contains the (107-111) sequence (known as osteostatin), a powerful inhibitor of osteoclastic activity (11,12) whose osteogenic properties will be discussed below in this editorial.
PTHrP is abundant in bone tissue, being present in the bone marrow hematopoietic cells, chondrocytes, and osteoblastic lineage cells (8). The importance of the osseous role of PTHrP has been described in mice with genetic manipulation of its gene. The comparison of mice with homozygotic suppression of the PTHrP (-/-) or PTH (-/-) gene leads to interesting results: while the latter mice showed bone dimorphism but are viable, the former mice showed severe chondrodysplasia with reduced endochondral development and excessive mineralization causing death by asphyxiation of these unborn mice (13). PTH (-/-) mice in the post-natal stage show more trabecular bone associated with a PTHrP increase. As a matter of fact, this increase disappears when they are crossed with PTHrP (+/-) mice (14). Mice with PTHrP haploinsufficiency are viable and show early osteoporosis at 3 months of age, which is characterized in the appendicular skeleton by a reduction in bone volume and changes in trabecular structure, increased osteoblastic apoptosis, and osteoprogenitor deficit in the bone marrow (15). In addition, the anabolic effect of intermittently administered PTH (1-34) was more significant in these PTHrP (+/-) mice (15). This suggests that the different bony levels of PTHrP could explain the variability observed in the anabolic response to teriparatide [commercialized PTH (1-34)] in osteoporotic patients. These findings are indicative that PTHrP is an essential factor for trabecular bone maintenance during growth. Bone PTHrP deficiency could contribute to low bone formation in involutional osteoporosis since its expression is reduced in the long bones of old mice and in primary human osteoblasts with the donor’s age (16,17). In addition, mutant mice that express a truncated PTHrP (1-84) show delayed growth, as well as bone apoptosis, early senescence, and osteopenia (18). More recently by transfecting osteoblastic cells with plasmids that express mutated forms of PTHrP, we were able to prove an effect of the NLS domain on osteoblast viability and osteoblastic differentiation (10). In addition, the C-terminal region of PTHrP has proven capable of inhibiting IL-1beta-induced senescence in primary human osteoblast cultures from patients with arthrosis (19). In vitro studies have also demonstrated the capacity of the C-terminal fragment of PTHrP —similar to that of the N-terminal fragment homologous to PTH— to increase osteoblastic viability in primary human osteoblasts (20). Interestingly, this effect of the C-terminal PTHrP fragment turned out to be strictly dependent on the transactivation of vascular endothelial growth factor receptor-2 (VEGFR2) (20,21). The anti-apoptotic effect of PTHrP in osteoblasts is particularly important because it is an essential element in the PTH anabolic action (22).
Using the ovariectomized mouse as an established model of primary osteoporosis, our group was able to prove a similar efficacy of both PTHrP N- and C-terminal peptides administered every two days for 4 to 8 weeks to improve the deteriorated trabecular structure in the femur by using micro-computed tomography (µCT); an effect associated with an increase in osteocalcin, a bone formation marker, and an inhibition of resorptive markers, including the expression of the SOST gene in bone tissue and pyridinoline residues resulting from degradation of type 1 collagen in plasma (23). Andy F. Stewart et al. pioneered the use of PTHrP (1-36) to study its efficacy in humans with primary osteoporosis. The daily injection of this peptide at doses greater than that of PTH (40 µg) for 3 months in post-menopausal women caused a similar bone mineral density increase in the lumbar spine with both peptides, although it was greater in the hip (a predominantly cortical bone) and in the femoral neck with PTHrP. In addition, PTH increased the N-terminal propeptide and C-terminal telopeptides of type 1 collagen, bone formation and bone resorption markers, respectively, while PTHrP (1-36) only affected the first marker (24). More recently, a peptide derived from PTHrP (1-36) has been synthesized with 10 amino acidic substitutions in its C-terminal end —abaloparatide— which has proved effective increasing bone mass with lower risk of hypercalcemia compared to teriparatide treatment (25). Therefore, in a stage 2 clinical trial in post-menopausal women with severe osteoporosis it was observed that abaloparatide was more effective compared to teriparatide increasing bone mineral density in extravertebral skeletal locations. In addition, the double-blind, multicenter stage 3 placebo-controlled clinical trial Abaloparatide Comparator Trial In Vertebral Endpoints (ACTIVE) has demonstrated the greater efficacy of abaloparatide at 18 months reducing the risk of spinal and non-spinal fractures in this situation. Abaloparatide has been approved by the FDA for the management of post-menopausal osteoporosis with high risk of fractures. The differences of action on the resorptive component between PTH and PTHrP are attributed to their interaction with different PTHR1 conformations: PTH predominantly with G protein-independent conformation (R0) resulting in a prolonged AMPc response that favors the resorptive component through RANKL; PTHrP with a G protein-dependent conformation that induces a shorter response, thus favoring its anabolic action (25).
Post-fracture bone regeneration can be compromised in osteoporosis and the current data indicate that systemic PTH is effective in this respect (26). Therefore, effectiveness of PTHrP was evaluated in an experimental bone regeneration model, medullary ablation in the tibia (27). Using osteoporotic mice treated with methylprednisolone, we demonstrated that the sequential administration (every 2 days) of PTHrP (1-36) or PTHrP (107-139) increased bone regeneration after medullary ablation (28). Taking this finding into account, we studied the possible osteo-regenerative effect of osteostatin, the sequence responsible for the anti-resorptive action of the C-terminal fragment of PTHrP (11), whose structural simplicity makes it especially attractive from a translational standpoint. Impregnation with osteostatin of mesoporous silica ceramics (SBA-15, synthesized and characterized by Prof. Vallet-Regí et al.) gives osteogenic properties to the biomaterial in murine osteoblast cultures of the MC3T3-E1 cell line (29). Implantation of this same material with osteostatin in a cavitary defect (that does not regenerate on its own) in the femoral epiphysis of healthy or osteoporotic rabbits induced new bone formation at 4-8 weeks in healthy animals (30), and at 2 weeks in osteoporotic rabbits (31). Subsequently, a biodegradable material (a gelatin-glutaraldehyde-coated hydroxyapatite polymer) was used impregnated with osteostatin or PTHrP (1-37) and implanted in a non-cavitary defect in the tibia of old osteopenic rats with or without diabetes mellitus. The presence of either PTHrP peptide in the graft induced the complete repair of the bone defect in a similar way at 4 weeks (32). An aspect particularly interesting in relation to osteostatin is that its in vivo anabolic action has been demonstrated in a diabetic mice model (with low bone formation). Dynamic bone histomorphometry demonstrated that treatment with equivalent doses of osteostatin or PTHrP (1-37) for 3 consecutive days normalized the decreased mineralize surface and the mineral apposition rate at 2 weeks, as well as bone formation, in the vertebrae of these mice (33).
In conclusion, PTHrP has turned out to be an essential factor for bone tissue development and maintenance. In addition, its osteogenic actions are not limited to its N-terminal region sharing structural similarities with PTH. The aspects discussed in this editorial have a special meaning considering the increased involutional osteoporosis associated with our longevity, which determines the growing demand for osteo-forming and osteo-regenerating molecules to repair fractures due to bone fragility. Therefore, basic and translational research have proven to be crucial to identify new PTHrP-based therapeutic strategies: in clinical use like abaloparatide or in potential development like osteostatin —a peptide derived from the C-terminal sequence of PTHrP— whose properties make it particularly attractive to promote bone formation and bone regeneration (Fig. 2).
BIBLIOGRAFÍA/REFERENCES
1. Hurtado J, Esbrit P. Treatment of malignant hypercalcaemia. Expert Op Pharmacother 2002;3:521-7. DOI:10.1517/14656566.3.5.521 [ Links ]
2. Moseley JM, Kubota M, Diefenbach-Jagger H, Wettenhall RE, Kemp BE, Suva LJ, et al. Parathyroid hormone-related protein purified from a human lung cancer cell line. Proc Natl Acad Sci USA 1987;84:5048-52. DOI:10.1073/pnas.84.14.5048 [ Links ]
3. Burtis WJ, Wu T, Bunch C, Wysolmerski JJ, Insogna KL, Weir EC, et al. Identification of a novel 17,000-dalton parathyroid hormone-like adenylate cyclase-stimulating protein from a tumor associated with humoral hypercalcemia of malignancy. J Biol Chem 1987;262:7151-6. [ Links ]
4. Strewler GJ, Stern PH, Jacobs JW, Eveloff J, Klein RF, Leung SC, et al. Parathyroid hormone-like protein from human renal carcinoma cells: structural and functional homology with parathyroid hormone. J Clin Invest 1987;80:1803-7. DOI:10.1172/JCI113275 [ Links ]
5. De Miguel F, Motellón JL, Hurtado J, Jiménez FJ, Esbrit P. Comparison of two immunoradiometric assays for parathyroid hormone-related protein in the evaluation of cancer patients with and without hipercalcemia. Clin Chim Acta 1998;277:171-80. DOI:10.1016/s0009-8981(98)00127-2 [ Links ]
6. Motellón JL, Jiménez FJ, de Miguel F, Jaras MJ, Díaz A, Hurtado J, et al. Parathyroid hormone-related protein, parathyroid hormone, and vitamin D in hypercalcemia of malignancy. Clin Chim Acta 2000;290:189-97. DOI:10.1016/s0009-8981(99)00181-3 [ Links ]
7. Martin TJ, Mosely JM, Williams ED. Parathyroid hormone-related protein: hormone and cytokine. J Endocrinol 1997;154:S23-37. [ Links ]
8. Philbrick WM, Wysolmerski JJ, Galbraith S, Holt E, Orloff JJ, Yang KH, et al. Defining the roles of parathyroid hormone-related protein in normal physiology. Physiol Rev 1996;76:127-73. DOI:10.1152/physrev.1996.76.1.127 [ Links ]
9. Gardella TJ, Vilardaga JP. International union of basic and clinical pharmacology. XCIII. The parathyroid hormone receptors —Family B G protein-coupled receptors. Pharmacol Rev 2015;67:310-37. DOI:10.1124/pr.114.009464 [ Links ]
10. García-Martín A, Ardura JA, Maycas M, Lozano D, López-Herradón A, Portal-Núñez S, et al. Functional roles of the nuclear localization signal of parathyroid hormone-related protein (PTHrP) in osteoblastic cells. Mol Endocrinol 2014;28:925-34. DOI:10.1210/me.2013-1225 [ Links ]
11. Fenton AJ, Kemp BE, Hammonds RG, Jr., Mitchelhill K, Moseley JM, Martin TJ, et al. A potent inhibitor of osteoclastic bone resorption within a highly conserved pentapeptide region of parathyroid hormone-related protein; PTHrP [107-111] Endocrinology 1991;129:3424-6. DOI:10.1210/endo-129-6-3424 [ Links ]
12. Ibáñez L, Nácher-Juan J, Terencio MC, Ferrándiz ML, Alcaraz MJ. Osteostatin inhibits M-CSF+RANKL-induced human osteoclast differentiation by modulating NFATc1. Int J Mol Sci 2022;23:8551. DOI:10.3390/ijms23158551 [ Links ]
13. Karaplis AC, Luz A, Glowacki J, Bronson RT, Tybulewicz VLJ, Kronenberg HM, et al. Lethal skeletal dysplasia from targeted disruption of the parathyroid hormone-related peptide gene. Genes Devel 1994;8:277-89. DOI:10.1101/gad.8.3.277 [ Links ]
14. Miao D, Jiarong L, Yingben X, Su H, Karaplis AC, Goltzman D. Parathyroid hormone-related peptide is required for increased trabecular bone volume in parathyroid hormone-null mice. Endocrinology 2004;145:3554-62. DOI:10.1210/en.2003-1695 [ Links ]
15. Miao D, He B, Jiang Y, Kobayashi T, Sorocéanu MA, Zhao J, et al. Osteoblast-derived PTHrP is a potent endogenous bone anabolic agent that modifies the therapeutic efficacy of administered PTH 1-34. J Clin Invest 2005;115:2402-11. DOI:10.1172/JCI24918 [ Links ]
16. Portal-Núñez S, Manassra R, Lozano D, Acitores A, Mulero F, Villanueva-Peñacarrillo ML, et al. Characterization of skeletal alterations in a model of prematurely aging mice. Age (Dordr) 2013;35:383-93. DOI:10.1007/s11357-011-9372-8 [ Links ]
17. Martínez P, Esbrit P, Rodrigo A, Alvarez-Arroyo MV, Martínez ME. Age-related changes in parathyroid hormone-related protein and vascular endothelial growth factor in human osteoblastic cells. Osteoporosis Int 2002;13:874-81. DOI:10.1007/s001980200120 [ Links ]
18. Miao D, Su H, He B, Gao J, Xia Q, Zhu M, et al. Severe growth retardation and early lethality in mice lacking the nuclear localization sequence and C-terminus of PTH-related protein. Proc Natl Acad Sci U S A 2008;105:20309-14. DOI:10.1073/pnas.0805690105 [ Links ]
19. Platas J, Guillén MI, Gomar F, Castejón MA, Esbrit P, Alcaraz MJ. Anti-senescence and anti-inflammatory effects of the C-terminal moiety of PTHrP peptides in OA osteoblasts. J Gerontol A Biol Sci Med Sci 2017;72:624-31. DOI:10.1093/gerona/glw100 [ Links ]
20. Alonso V, de Gortázar AR, Ardura JA, Andrade-Zapata I, Alvarez-Arroyo MV, Esbrit P. Parathyroid hormone-related protein (107-139) increases human osteoblastic cell survival by activation of vascular endothelial growth factor receptor-2. J Cell Physiol 2008;217:717-27. DOI:10.1002/jcp.21547 [ Links ]
21. Esbrit P, Alcaraz MJ. Current perspectives on parathyroid hormone (PTH) and PTH-related protein (PTHrP) as bone anabolic therapies. Biochem Pharmacol 2013;85:1417-23. DOI:10.1016/j.bcp.2013.03.002 [ Links ]
22. Jilka RL, Weinstein RS, Bellido T, Roberson P, Parfitt AM, Manolagas SC. Increased bone formation by prevention of osteoblast apoptosis with parathyroid hormone. J Clin Invest 1999;104:439-46. DOI:10.1172/JCI6610 [ Links ]
23. De Castro LF, Lozano D, Portal-Núñez S, Maycas M, de la Fuente M, Caeiro JR, et al. Comparison of the skeletal effects induced by daily administration of PTHrP (1-36) and PTHrP (107-139) to ovariectomized mice. J Cell Physiol 2012;227:1752-60. DOI:10.1002/jcp.22902 [ Links ]
24. Horwitz MJ, Augustine M, Kahn L, Martin E, Oakley CC, Carneiro RM, et al. A comparison of parathyroid hormone-related protein (1-36) and parathyroid hormone (1-34) on markers of bone turnover and bone density in postmenopausal women: the PrOP study. J Bone Miner Res 2013;28:2266-76. DOI:10.1002/jbmr.1978 [ Links ]
25. Ardura JA, Portal-Núñez S, Alonso V, Bravo B, Gortázar AR. Handling parathormone receptor type 1 in skeletal diseases: realities and expectations of abaloparatide. Trends Endocrinol Metab 2019;30:756-66. DOI:10.1016/j.tem.2019.07.014 [ Links ]
26. Hong H, Song T, Liu Y, Li J , Jiang Q, Song Q, et al. The effectiveness and safety of parathyroid hormone in fracture healing: A meta-analysis. Clinics (Sao Paulo) 2019;74:e800. DOI:10.6061/clinics/2019/e800 [ Links ]
27. Ono N, Nakashima K, Schipani E, Hayata T, Ezura Y, Soma K, et al. Constitutively active PTH/PTHrP receptor specifically expressed in osteoblasts enhances bone formation induced by bone marrow ablation. J Cell Physiol 2012;227:408-15. DOI:10.1002/jcp.22986 [ Links ]
28. De Castro LF, Lozano D, Dapía S, Portal-Núñez S, Caeiro JR, Gómez-Barrena E, et al. Role of the N- and C-terminal fragments of parathyroid hormone-related protein as putative therapies to improve bone regeneration under high glucocorticoid treatment. Tissue Eng Part A 2010;16:1157-68. DOI:10.1089/ten.TEA.2009.0355 [ Links ]
29. Lozano D, Manzano M, Doadrio JC, Salinas AJ, Vallet-RegíM, Gómez-Barrena E, et al. Osteostatin-loaded bioceramics stimulate osteoblastic growth and differentiation. Acta Biomater 2010;6:797-803. DOI:10.1016/j.actbio.2009.08.033 [ Links ]
30. Trejo CG, Lozano D, Manzano M, Doadrio JC, Salinas AJ, Dapía S, et al. The osteoinductive properties of mesoporous silicate coated with osteostatin in a rabbit femur cavity defect model. Biomaterials 2010;31:8564-73. DOI:10.1016/j.biomaterials.2010.07.103 [ Links ]
31. Lozano D, Trejo CG, Gómez-Barrena E, Manzano M, Doadrio JC, Salinas AJ, et al. Osteostatin-loaded onto mesoporous ceramics improves the early phase of bone regeneration in a rabbit osteopenia model. Acta Biomater 2012;8:2317-23. DOI:10.1016/j.actbio.2012.03.014 [ Links ]
32. Ardura JA, Portal-Núñez S, Lozano D, Gutiérrez-Rojas I, Sánchez-Salcedo S, López-Herradón A, et al. Local delivery of parathyroid hormone-related protein-derived peptides coated onto a hydroxyapatite-based implant enhances bone regeneration in old and diabetic rats. J Biomed Mater Res A 2016;104:2060-70. DOI:10.1002/jbm.a.35742 [ Links ]
33. Maycas M, McAndrews KA, Sato AY, Pellegrini GG, Brown DM, Allen MR , et al. PTHrP-derived peptides restore bone mass and strength in diabetic mice: Additive effect of mechanical loading. J Bone Miner Res 2017;32:486-97. DOI:10.1002/jbmr.3007 [ Links ]











 texto en
texto en