INTRODUCTION
Probiotics are defined as live microorganisms, which beneficially affects the host physiology by improving the intestinal microbial balance in the gastrointestinal tract1,2. Their perceived human health beneficial effects including: prevention and treatment of gastrointestinal disorders, colorectal cancer prevention, antitumor activity and prevention of genital and urinary tract infections by inhabitation of Candida albicans3. In addition, their benefits comprise modulation of the immune system, curative effects on several types of diarrhea of different etiologies, reducing the bad and total cholesterol in the blood, improving brain function, control and maintenance of the vaginal lactic acid bacterial microbiota and preventing colonization ‘undesirable’ organisms4.
The survivability of the population of these microorganisms is affected by many factor that the pH is the main factor that have been claimed to affect the viability of probiotic cultures. To obtain a positive probiotic effects, the microorganisms must survive the transit through the highly gastric acidic condition and reach the small intestine in a viable state5,6. Encapsulation as a technique for improving the survival of probiotics and controlled and continuous delivery of them into the human gut has been studied7. The goal of this technology is to maintain greater cell viability despite the stomach acidity8. Additionally, this technology is capable to stabilize cells, enhance their viability and potential stability during production, storage, handling and consumption9.
In the most reports, Alginate (Alg) is a base material for encapsulation procedure. Alg is a natural water soluble polysaccharide exhibiting excellent biocompatibility and biodegradability. It is readily beneficial for application in three-dimensional structure10,11. Divalent cations such as Ca2+ can interact ionically with glucuronic acid blocks in Alg, causes to gelation is occurring and formation of three-dimensional network 12. Alg is water-swollen polymer and swelling and deswelling properties of Alginate is sensitive to the environmental pH. This property is very important for controlling release encapsulated materials in colon condition10. The swelling capacity of hydrogels is one of the most important parameters that evaluate the properties of pH-sensitive hydrogels and the swelling behavior of them depends on pH. This property has great importance for controlling the encapsulated materials releasing in colon condition.
Egg white proteins (EWP) are extensively utilized in a variety of food ingredients due to their superior gelling and emulsification properties 13. The use of EWP is favored, because of its characteristics; low cost, simplicity, bio compatibility and nutritional content 14. Although it is well known for the protection of bacteria during gastric passage need to be buffering occurs 15. EWP peptides have strong functionality, reactivity and amphoteric properties because of the presence of carboxyl, amino and other chemical groups, such as: –OH, –CONH–, –CONH2, –SH, –SO3H and phenolic groups 16. The physicochemical properties of proteins promise the application of them to stimuli-response system as novel controlled delivery of biologically-active substances 17. EWP is insoluble at gastric pH condition, this property is essential for probiotic encapsulation, because the biomaterial must not dissolve into the stomach, but only in the gut.
The goal of this study was to develop a new and simple method for encapsulation of probiotics based on an extortion technique by means of egg white proteins and Alginate and use of their ability to protect probiotic cells during exposure to pH-conditions of the human stomach. Furthermore, the physicochemical properties of the resulting capsules, pH-sensitivity, swelling propriety and morphology were investigated.
MATERIALS AND METHODS
Preparation of microorganisms
Lactobacillus casei ATCC39392 and Lactobacillus acidophilus ATCC39456 were prepared as lyophilized form Microbiology Laboratory, Drug Applied Research Center, Tabriz University of Medical Sciences, Iran. A vial of freeze dried L. casei, and L. acidophilus were inoculated into 10 ml MRS broth (De Man, Rogosa and Sharpe broth, Merck, KGaA Germany) and incubated at 37 °C for 48 hours (activation time of L. casei) under anaerobic conditions. Then the cultures were sub-cultured into 95 ml broth and incubated under previous condition. The cells were harvested by using cold centrifuging (4°C) at 4500 g for 10 min and washed twice with sterile peptone water 0.1% (Merk, KGaA Germany).
EWP powder prepared by drying 1000 ml liquid egg white in 4°C at sterile conditions. The EWP powder contained 54% Ovalbumin, 12% Ovotransferrin, 11% Ovomucoid, 4% Ovoglobulin G2, 4% Ovoglobulin G3, 3.5% Ovomucin, 3.4% Lysozyme and other materials 18, Lysozyme is the bacteriolytic substance in egg white which is isolated from this powder. Fifty gram of the EWP powder dissolved in 1 liter sterile distilled sodium hydroxide water solution (adjusted in pH=10.5) to make 5% (g/100ml) EWP solution. Lysozyme has a lower solubility in that pH and may be crystallized directly from egg white 19. In order to remove lysozyme and Salmonella Enteritidis, this solution crossed flow microfiltration (MF) for removal of a cocktail of Salmonella enterica species from egg white solution 20. Then filtered solution was neutralized by hydrochloric acid water solution and adjusted to pH 7.0.
Encapsulation of microorganisms
To make 7% (g/100 ml) solution, 1.4 g of the EWP powder was dissolved in 20 ml sterile distilled water and maintained for 24 hours in refrigerator at 0 °C for complete hydration of the proteins. Medium viscosity sodium alginate powder (Sigma Aldrich, Pool, UK) was dispersed in distilled water to make 3% (g/100ml) solution, then autoclaved in (120 °C for 15 min) and stirred for 30 min and stand overnight in refrigerator. EWP and Alginate solution were combined to form a solution with EWP/Alginate ratio of 1:1(v/v) with vortex for 30 min sodium alginate-EWP capsules were produced by mixing 18 g EWP-Alginate solution (1:1 v/v), with 1-1.5 g washed bacteria suspension. The resulting solutions were injected into 100 ml containing 2g/100ml calcium chloride water solution (CaCl2, Merck, Germany) and gelation was started by extrusion. The EWP-Alg capsules were formed during continuously stirring for 20 min. The capsules were allowed to stand for 30 min to complete gelation, then rinsed and subsequently kept in sterile peptone solution 0.1% at 4°C. Then centrifuged at 500 rpm for 5 min at 4 °C. Magnetic stirring was carried out for 15 min. The resulting capsules washed twice with distilled and deionized water.
In other process 10 ml distilled water added to 10 ml of sodium alginate solution that previously has been produced (3%) to make a 1.5% (g/100ml) solution and then mixed with 1-1.5g bacteria suspension. This solution was loaded into 5 ml plastic syringes equipped with insulin syringe needle and gelation was started by extrusion into 100 ml containing 2 g/100 ml calcium chloride water solution (CaCl2, Merck, Germany). The capsules were dried in sterile conditions and at 4 °C for 48 h.
Survival assays, release and bacterial count
Counting the free and encapsulated bacteria
The enumeration of free cells of L. acidophilus and L. casei as a control were dispersed in a mixed sterile solution of Sodium Citrate (1.0%w/v) mixed by vortex for a few minutes and was assayed by a serial 10-fold dilution. 0.1 ml of the samples was plated on MRS agar (Merck). Colony forming units (CFU) were determined after 48-72 h anaerobic incubation at 37°C and the counts were reported as mean log CFU. mL-1.
For counting the encapsulated probiotics and viability of them in freshly prepared capsules of Calcium Alginate (0.1g) and EWP-Alg (0.1g), they were liquefied in 10 ml of 1% (w/v) sterile Sodium Citrate solution (Merck) (pH=6.0) by shaking gently at room temperature using a magnetic stirrer at 37°C for 15 min. L. acidophilus and L. casei by a serial 10-fold dilution were counted triplicate in MRS agar (Merck) at 37°C for 46-72h.
Evaluation of Survival of bacterial cells in simulated gastric juice
For evaluation of the survivability of free cells at a low pH-value of simulated gastric conditions, 0.1 ml washed cell suspensions were inoculated into 10 ml of simulated gastric juice (containing 0.2% NaCl, 0.08 M HCl; pH 1.5, temperature 37°C) without pepsin 21. All three samples were mixed well, incubated at 37°C and sampled 45, 90, and 135 min after addition of the bacteria. Then bacteria cells were precipitated by centrifugation and were dispersed in 10 ml sterile solution of Sodium Citrate (1.0%w/v). Surviving bacteria were counted by pour plate method in MRS agar after incubation at 37°C.
Determination of the protective effect of the encapsulation process on the survivability at low pH-values of simulated gastric conditions at 37°C was done as described by Rao et al. (1989) 21. Simulated gastric juice, without pepsin was prepared according to the standard conditions given by US-Pharmacopeia (2008), consisted of 9 g/l Sodium Chloride (NaCl) (Merck), with pH adjusted to 1.5 with HCl. Freshly and freeze dried encapsulated (0.1g) L. acidophilus and L. casei were completely liquefied in 10 ml of previously prepared sterile simulated gastric juice and were incubated at 37°C for 1, 2, and 3 hour. After incubation, the sampled capsules were removed by the filter and neutralized with sodium hydroxide (NaOH) solution and washed with 0.1% peptone solution. Previously acid-incubated capsules were dispersed in 10 ml 1% (w/v) sterile sodium chloride solution and the counts of the viable cells of each bacterium were counted in triplicates as the method described in the previous section.
Morphology of EWP- Alginate capsules by Scanning Electron Microscope
The morphology and surface structure of the natural, acid induced capsules and cross-section of them were recorded with scanning electron microscope photographs taken with (MIRA3 FEG-SEM instrument, TSECAN, Czech Republic) at an accelerating voltage of 17.13 kV22. The capsules were made conductive by sputtering thin coat of gold under vacuum and then the images were recorded.
Fourier Transform Infrared Spectroscopy (FTIR)
FTIR spectra of the dried, semi moisture trace and acid induced capsules were recorded at room temperature in the frequency range of 400 - 4000 cm-1 on a KBr Press Model SHIMADZU FTIR-5300. Samples were thoroughly grounded with exhaustively dried KBr and pellets were prepared by compression under vacuum and their corresponding FTIR spectra were recorded 23.
PH-sensitive Swelling and Deswelling of Hydrogel Particles
The swelling and deswelling behaviors of the pH-sensitive hydrogel capsules were investigated at various pHs by gravimetry at 37°C. The clean and pre-weight dried capsules of hydrogels were soaked in distilled water for different interval time durations to reach the equilibrium state. The capsules were removed and the excess surface water was slightly removed by filter paper. The swelling ratios in different pH were calculated as follows: swelling ratio or SR = (Ws–Wd)/ Wd, where Wd was the weight of dried sample, and Ws was the weight of swollen samples at different time and equilibrium swollen or ES = (W∞–Wd)/Wd, where W∞ is the weight of equilibrium swollen hydrogels. The swelling experiments on hydrogels of this study have been performed in different pH solutions (1.5, 4.0, 7, and 8.5) at 37°C (±0.5°C).
The deswelling behavior of hydrogel capsules and pH sensitivity were studied that were previously maintained at solution in different pH (pH=7.0 as neutral pH), pH=1.5 (Simulated gastric juice condition) and pH=8.3 simulated intestinal juice (0.05 M KH2PO4, pH 7.00)) and then dried at 25 °C by measuring these capsules weight at different time. Deswelling ratio was calculated by the equation: deswelling ratio = (Wt–Wd)/Wd, where Wt was the weight of capsules at a given time during deswelling, Wd was the weight of dried capsules. And when t = 0, Wt was the weight of swollen hydrogel at beginning of deswelling.
RESULTS
Counting of encapsulated bacteria
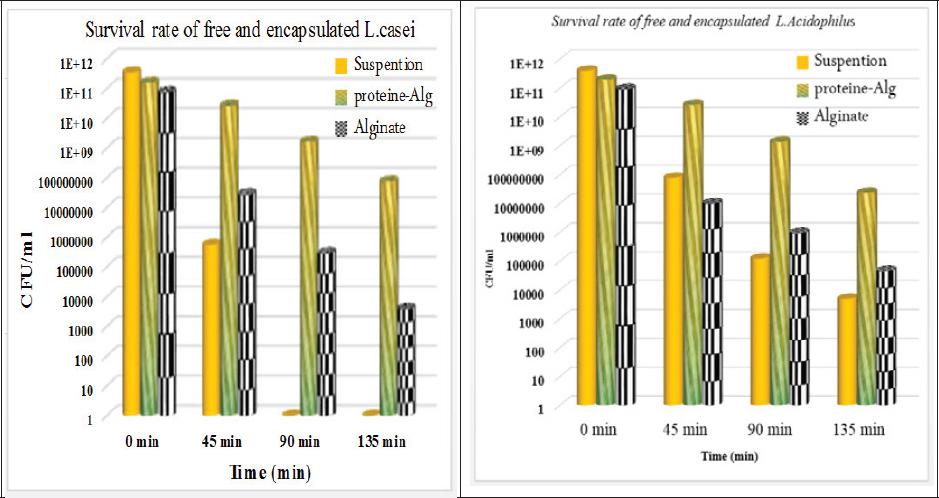
The amount of L. casei and L. acidophilus free population were 3.61 × 1011 and 4.06 × 1011, respectively, and after encapsulation by Alginate were 7.93 × 1010 and 9.64 × 1010, respectively. The results of encapsulation with EWP- Alg showed that encapsulated L. casei and L. acidophilus were 1.61 × 1011 and 1.96 × 1011, respectively, which were shown in the figure 2.
Survival results in the simulated gastric condition
The protective effect of hydrogels containing the bacteria with the aim of improving the viability of the passage of simulated gastric conditions (pH = 1.5) was investigated. Probiotics as encapsulated with Alginate, EWP-Alg and free cells as a control, were exposed at the simulated gastric conditions at the time of zero, 45, 90 and 135 minutes. According to the figure 2, the evaluation of free L. casei and L. acidophilus showed that the number of encapsulated bacteria after 45 minutes exposure to the gastric same solution was as the 5.66 × 106 and 7.81 × 107, respectively. The count after 90 minutes has declined to zero and 1.24 × 105 and after 135 minutes of test time reduced to zero and 5.00 × 103, respectively. The results showed that the sensitivity of the L. casei is more than L. acidophilus against stomach acid. While, according to most reports, the beneficial effects of L. casei were more than L. acidophilus in the intestinal environment 3.
Counting the number of Alginate coated L. casei and L. acidophilus showed that after 45 minutes of exposure to simulated gastric conditions declined to 2.94 × 107 and 1.03 × 107, respectively. Also, after 90 minutes reached to 3.02 × 105 and 9.68 × 105 and after 135 minutes during the test period was reduced to 4.00 × 103 and 4.7 × 104.
The results of protective effect against the stomach acidic conditions by coating with EWP-Alg showed that the number of alive bacteria in the EWP-Alg capsules after 45 minutes exposure to gastric simulation solution declined to 2.74 × 1010 and 4.62 × 1010 for L. casei and L. acidophilus, respectively. In addition, after 90 minutes reached to 1.68 × 109 and 8.13 × 109 minutes, and 135 minutes after the test time declined to 7.75 × 107 and 2.34 × 107, respectively. These results suggest that coating with EWP- Alg has significant effect on the probiotics viability through the acidic conditions of the stomach and has better performance than Alginate. In addition, encapsulation in EWP- Alg capsules greatly improved the survival rate of L. casei and L. acidophilus compared to free cells and encapsulated in Alginate under simulated gastric condition. After 90 min Survival rate in the EWP- Alg - capsules were 1.04% and 0.70% for L. casei and L. acidophilus, respectively 24.
The morphology of Alginate and EWP- Alginate capsules
Scanning electron microscopy (SEM) of Alg and EWP-Alg capsules are shown in Figure 1 (A, B, C and D). The SEM images showed that the morphology of the Alg capsule was very affected by the acidity of the simulate solution of stomach conditions and the crystal structures have been destroyed by acid. Thus, its less productivity than the EWP-Alg can be due to this phenomenon. As compared with the structure of EWP-Alg in the exposure with acid, Alginate does not greatly changed and is still retained their morphology. However, small changes can be seen. Capsules of EWP-Alg after exposing with acid was more uniform and the number of cavities reduced and these indicates the response of the polymer to the acid, most likely due to the cross linking after exposure to gastric acid. Three-dimensional structure of most proteins, particularly the EWP, in the face of strong acids has changed and molecular interaction causes cross linking or denaturation and sediment 25.
The results of Fourier transform infrared spectroscopy (FTIR)
The range related to control capsules of EWP- calcium Alginate (EWP-Ca-Alg) (Figure 3), absorption in the wavelength 3410 cm-1 is related to OH hydrogen bond, which a broad peak with relatively high intensity can be seen. In addition, in the area of 2919/8 cm-1 and 2919/0 cm-1, absorption is related to aliphatic C-H. The peak in the 1661cm-1 is associated with asymmetric stretch vibration of COO- and the peak in the 1436 cm-1 is related to symmetric stretch vibration of COO-. In addition, the peak of 1031 cm-1 is related to stretch vibration area of C-C, C-O and C-O-C.
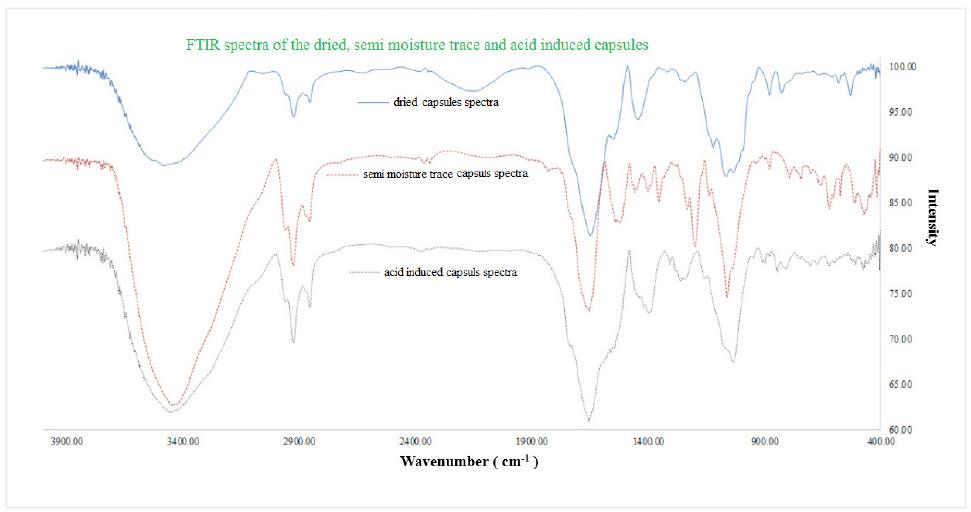
Figure 3 Fourier transform infrared spectroscopy (FTIR) of fresh prepared acid induced and heated Alginate-EWP capsules
FTIR spectra of control, heated and acid induced calcium Alg –EWP has been shown in Figure 3. Comparison of the spectra showed that the peak of absorption in 3410 cm-1 was related to OH hydrogen bond, which in comparison with similar peak of control calcium Alg- EWP capsule, was wider and had much more intensity. This could be due to interaction between functional groups, NH2 or N-H on protein chains with COOH functional groups of the Alginate polymer.
Peak of 599 cm-1 related to EWP, due to intermolecular interactions between networked Alg and the protein chain had a very wide range and poorly could be seen. The peak of 755 cm-1 due to intermolecular interactions between the Alg polymer and EWP had wide spectrum and moved to absorption band of 795 cm-1. Absorption bands (NH bending vibrational) of 882 cm-1 in the spectrum of unheated and not acid induced EWP-Alg capsules were seen. The peak in the spectrum of the heated and acid induced capsules has been moved to 838 cm-1 that the movement to 838 cm-1 maybe due to engaging of NH2 or N-H functional groups with calcium ion of the polymer26.
Intense peak in the region of 915 cm-1 till 1130 cm-1 in the spectrum of control calcium Alg-EWP was related to the C-O frequency absorption band that the peak in the spectrum of heated and acid induced calcium Alg-EWP also were seen. The peak moved to higher wave and the peak width was reduced. This was because of bonding of proteins O atoms to the Alginate calcium ion, resulting release of CO link, more resonant than the initial state and enhance the character of π CO. The peak of 1215 and 1254 cm-1 was related to C-C and CCO bending and C-O tensile strength, respectively that presented in the polymer Alg and protein. Their locations were in a spectrum of -CH2- bending that showed to peak of 1255 cm-1 represented the interaction between the Alg polymer and protein.
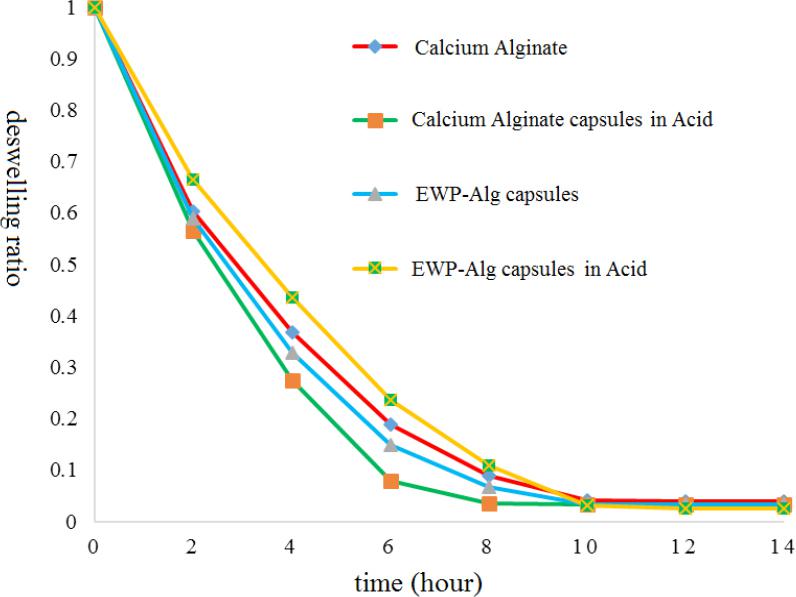
The results of sensitivity of capsules to acid by swelling and deswelling.
Inflationary and contractionary behavior of capsules in different pH conditions at 37°C was examined. As seen in the figure 4, capsules that made of EWP- Alg in the distilled water and neutral condition swelled quickly to 3.27 times of their own weight absorb water. Also, after about an hour to get on the simulated gastric acidity conditions (pH = 1.5), about 2.43 times of its own weight absorbed water. In these circumstances, swelling capacity of the capsule was reduced and this might be due to interactions between the Alg anionic polysaccharide and protein chains under the isoelectric point of these proteins. Exposure in the simulated intestines alkaline condition (pH = 8.3) increased the hydrogel swelling capacity and in these circumstances, capsules to 3.7 times their weight absorbed water for about an hour. It was due to the conversion of the carboxyl group (COOH) on the Alg polysaccharide chains to carboxylate groups (COO-) at pH above 7 27.
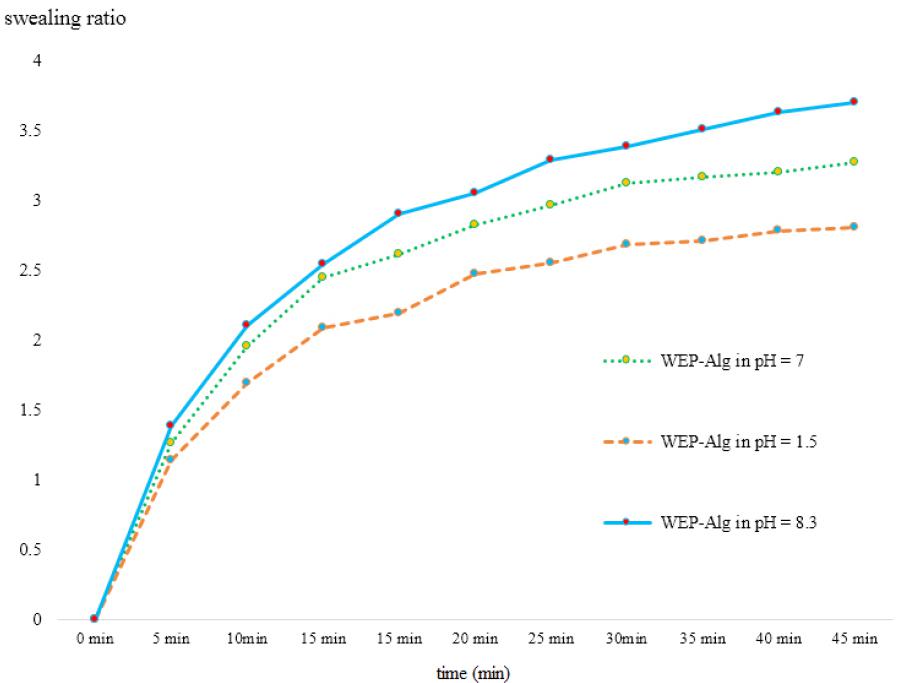
Figure 4 sensitivity to acid by swelling ratios of fresh prepared acid induced and Alginate-EWP capsules in different environment pH.
As seen in Figure 5, the weight loss slopes of calcium Alg capsules in drying of acid induced calcium Alg were rather than the slope of capsules were exposed to distilled water at neutral pH. This behavior could indicate high rate of water loss in the form of calcium Alg capsules, and this phenomenon might be due to the relatively low number of network connections in the acid induced capsule, which was created by calcium ions between the Alg chains.
DISCUSSION
In dissolution test of capsules, the dissolution of acid induced EWP-Alg capsules only in Sodium Citrate 0.5% -sodium sulfite 0.5 percentage solution. It can be due to occurring of chemical disulfide bonds between hydrogen sulfide as a chemical agent on the protein chains during immersed in acidic solution. The results of viability of probiotic cells entrapped in encapsulation with Alginate and EWP-Alg capsules represented that there was no significant loss of viability for all strains. Also the results of survival of encapsulated cell in the simulated gastric condition were showed that these results are in accordance to in-vivo trials by Heidebach et al. (2009), who found a higher gastric survival rate of Bifidobacterium lactis and L. paracasei after 90 min with about 0.01% by microencapsulation of probiotic cells by means of rennet-gelation of milk proteins 28. Also, for L. casei a higher survival rate of cells was achieved compared with Chen et al who investigated activity of encapsulated L. bulgaricus in Alginate-whey protein microspheres 29. The proteins can be easily mixed with negatively charged polysaccharides such as Alg, carragenate or pectin 30, 31. When the pH is adjusted below their isoelectric point, the net charge of the proteins becomes positive, causing an interaction with the negatively charged polysaccharides 32. These results suggested encapsulation by using egg white proteins (EWP) is good choice for encapsulation of probiotic cells rather than Alg, chitosan, starch and gelatin had been used by many researchers 25.
Investigation of the morphology of Alg and EWP- Alg capsules was concluded that the strong acids caused denaturation of EWP and its sedimentation. When the acidity of the solution was below the isoelectric point, due to interactions between the protein chains and Alg carboxyl groups, caused the cross linking of the protein and Alg chains.
The results of the FTIR were showed interaction between proteins and Alg, when the pH were under isoelectric point of proteins in gastric acidity condition.
From the result of sensitivity of capsules to acid by swelling and deswelling studies, we can conclude that the breach of network links between calcium Alg chains were broken by the acidity of the environment and reduced the capacity of the polymer is water conservation. While such a comparison chart between the slopes of the weight loss, EWP-Alg capsules at neutral pH and acid induced EWP-Alg capsule showed different results. In this case, the slope of the capsules of EWP-Alg exposed to acid was lower than capsules EWP-Alg exposed to distilled water at neutral pH. This phenomenon might be due to the formation of bonds between chains of protein and Alg chains due to reduced pH below the isoelectric point of the protein exposed to simulated gastric acidity in the same solution33.
CONCLUSION
From the present study, it can be concluded that the use of egg white proteins-Alg for encapsulation of probiotics enhanced the stability of these microorganisms in simulated gastric environment adverse conditions. The results showed that the loss of cells during the encapsulation process was not significant and the high potential of this type of formulation had good improvement in the bacterial survival of the probiotic bacteria. A recent study has indicated that the survival of Alg immobilized bacteria was not significant and this may be due to destroying of calcium Alg crystal structures by the acidity of the gastric acid. The swelling and deswelling behavior of capsules that made of EWP- Alg in different PH showed that the swelling of the capsule was affected by pH. This study introduced a new method for encapsulation of probiotics with safer delivery to intestine.