INTRODUCTION
Quercetin with a structure of (3,3 ‘, 4’, 5,7-pentahydroxy flavone) is a kind of flavonoids and a sub-branch of polyphenols.1 The common sources of quercetin are: onion, apples, tomatoes and red wine.2 It exerts multiple pharmacological effects, including potent anti-oxidant activity, induction of apoptosis, modulation of cell cycle anti-mutagenesis, inhibition of angiogenesis and anti-inflammatory effects. Recently it has been reported that topical application of quercetin inhibits oxidative skin damages and the inflammatory processes induced by solar UV radiation.3 Topical use of quercetin is frequently hampered by its low skin permeability and poor solubility in aqua media, which make the development of pharmaceutical formulation difficult. Different strategies, such as prodrug4 and micro emulsion5, have been used to improve quercetin topical delivery. Quercetin is one such flavonoids which delay oxidant injury and cell death by scavenging oxygen radicals6, protecting against lipid peroxidation7 and thereby terminating the chain radical reaction, chelating metal ions, to form inert complexes that cannot take part in the conversion of superoxide radicals and hydrogen peroxide into hydroxyl radicals.8 studies of the topical application of compounds with free radical scavenging improve wound healing and protect tissue from oxidative damage.9
Liposomes are microscopic vesicles with an aqueous core surrounded by outer shell composed of phospholipids in a bilayer. They can incorporate a variety of hydrophilic and hydrophobic drugs, improve the accumulation of the drug at administration site, and reduce side effects.10-13 Hence, liposomes have been widely used as safe and effective drug vehicles in topical applications.14-17 liposomes offer a number of advantages in dermal and transdermal drug delivery as they have a high solubilization capacity and a penetration enhancer effect.18 The skin is the most superficial layer of the body, which consists of three layers of epidermis and dermal and hypoderm. The main action of the skin is a protective barrier against environmental contaminants. Collapse of skin integrity resulting from physical damage can result in illness and even death. Skin is very often prone to oxidation, wounds and burns caused by environmental factors, pathological conditions or external trauma. Poor healing of such skin damage may result in serious complication and possible development of chronic skin lesions.19,20 the healing of chronic wound is a complex process that involves more than one physiopathological factor, such as inflammatory response, over production of destructive proteases and oxygen free radicals.21 A compression scars called “subcutaneous wounds” is created in patients with absolute rest and in people who have been in the bed for a long time. The main cause of the ulcer of the bed is due to prolonged stress on the skin and subsequent lack of blood supply to the organ, and if developed, it can involve muscle and soft tissue and even bone.22 In this study, a liposomal formulation prepared by the fusion method and characterized as a candidate for topical delivery of quercetin.
MATERIAL AND METHODS
Materials
Soy phosphatidylcholine (phospholipon 85G) was obtained from lipoid (Germany). Quercetin, cholesterol and HEPES (4-(2-Hydroxyethyl)1-piperazine ethanesulfonic acid) were purchased from Sigma (Germany). Propyl paraben, methyl paraben, propylene glycol and vitamin E were obtained from Merck (Germany).
Preparation of liposomes
Liposomes were prepared by the fusion method in different concentration of quercetin. Briefly, the lipid components consisted of Soy phosphatidylcholine (SPC) (20%), cholesterol (2%), propylene glycol (3%), vitamin E (0.3%), methyl paraben (0.1%), propyl paraben (0.02%) were melted at about 75 °C. HEPES buffer (10Mm, pH 5) containing propylene glycol and quercetin in final concentration of 0.002, 0.004 or 0.008g/ml was heated separately and was added up to 100% to the previously heated melted lipids, and the mixture homogenized with a homogenizer (Ultra-Turax IKA T25) for 5 min at 12,000 rpm and allow it to cool down to room temperature.23
Characterization of the liposomes
Particle size
The particle size of the samples were measured in triplicate by laser light scattering (Scatterscope 1, Qudix, South Korea). Samples were diluted in HEPES buffer to a suitable concentration (0.2 g formulation in 1 ml HEPES buffer).
Incorporation efficiency
Incorporation efficiency of liposomes was determined directly. Certain amounts of liposomal dispersions were centrifuged (VS-35SMTI, Korea) at 20,000 rpm for 25 min at 25°C. Precipitated pellets were re-dispersed in 2 ml triton X-10 and the final clear solution was analyzed for quercetin content using a UV spectrophotometer at 369 nm.24
In vitro drug release
Drug release studies were performed using dialysis membrane method. Dialysis membranes were soaked before use in distilled water for 20h. 1g of formulation was placed in a dialysis membrane and both ends were closed. The membrane was float in a beaker containing 150 ml phosphate buffer (pH 7.4) and ethanol (3:1 v/v) and stirred at 200 rpm in 37 °C. 1 ml of receiver medium was removed at 15, 30, 45, 60, 90, 120, 180, 240, 360 and 480 min and same volume of fresh medium was replaced. The samples were analyzed for their quercetin content. The derived concentration values were corrected by using the equation:25
Mt (n) =Vr X Cn+ Vs*? Cm
Mt (n) is the current cumulative mass of drug transported across the membrane at time t, n is the number of sampling, Cn is the current concentration in the receiver medium ,Cm is the summed total of the previously measured concentration, Vr is the volume of the receiver medium, and Vs corresponds to the volume of the sample removed for analyzed26,27.
Stability
The formulations were stored at refrigerate (4 °C) for 3 months and the particle size and incorporation efficacy of the formulations were measured and compared with those at the time of preparation28.
Statistical analysis
All experiments were repeated three times and expressed as the mean ± standard deviation. One way analysis of variance (ANOVA) followed by multiple comparisons Tukey test was used to substantiate statistical differences between groups. Result with P < 0.05 were considered to be significant.
RESULTS
The aim of the present work was to investigate the ability of new nanovesicle, to load quercetin and improve its local bioavailability. In this study the fusion method was used to prepare the topical liposomal formulations. The method is free of organic solvent and yield homogeneous liposomes with high incorporation efficacies. All experiments were repeated three times.
Characterization of the liposomes
Particle size
The mean particle size of the liposomal formulations was shown in Table 1. The larger the amount of drug used in the formulation, the larger the particle size. ANOVA analysis showed statistical significant differences between F1 (0.002 g/ml) and F2 (0.004g/ml) (P<0.05) and also F1 (0.002 g/ml) and F3 (0.008 g/ml) (P<0.05).
Incorporation efficiency
The incorporation efficiency of formulations was in range of 80.55 to 96.80% (Table 1).T test analysis showed statistical significant differences between F1 (0.002 g/ml) and F2 (0.004 g/ml) (P<0.05). F3 was discarded from the rest of the study because of the sedimentation of un-loaded drug after centrifuge. The lipophilic nature of quercetin may lead to incorporation of this drug in between the lipid bilayers. This can explain the high incorporation efficiency of the quercetin in liposomal formulations.29
In vitro drug reléase
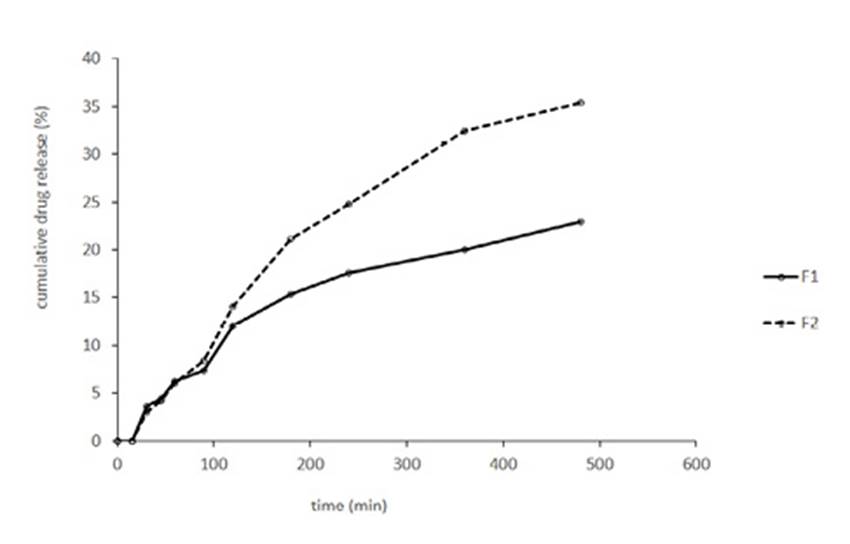
The release profile of quercetin from various liposomal formulations was shown in Fig. 1. T test analysis showed statistical significant differences between F1 (0.002 g/ml) and F2 (0.004 g/ml) from 90 min till 480 min (P < 0.05). At the end of the test F1 released 22.97±0.01% of quercetin and F2 released 35.37±0.005% of that. But by assay of quercetin at the end of the test, it was assumed that 58.23±0.94% of quercetin in F1 and 50.06±2.39 % of quercetin in F2 has been remained in dialysis bag. So it can be concluded that about 15% of released quercetin has been adsorbed by the surface of container and/or dialysis bag.
Stability
The results of stability study for formulations are shown in Table 2. Over the course of 3 months, the mean particle size of F1 changed significantly (P<0.05). F1 at the time of preparation showed very low particle size ( less than 10 nm) and this matter could be the reason of increasing particle size during 3 month storage, whereas F2 remained stable in case of particle size. This matter could be resulted by the presence of cholesterol that has stabilizing effect against aggregation and fusion of the liposomes.30 Incorporation efficacy of formulations did not significantly changed during 3 month storage that may be due to the lipophilic nature of quercetin that intended to be remained in lipid vesicles.
DISCUSSION
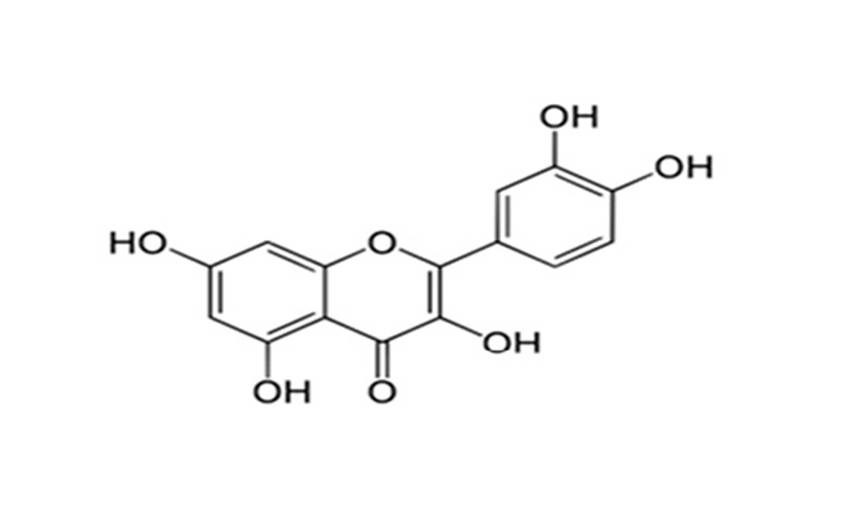
The chemical structure of polyphenolic quercetin is reported in Fig. 2. Quercetin is a yellow-green, polyphenolic flavonol containing the 3-hydroxyflavone backbone with five oxydrilic group in position 3’-4’ and 3-5-731. In the present investigation, effective phospholipid nanovesicular formulation were developed to achieve maximum loading capacity and drug release.
Particle size has a key role in other characteristics of nanovesicles such as liposomes. The particle sizes of liposomes are shown in Table 1. As shown in Table 1, the higher concentration of quercetin in the formulation has led to increase the mean particle size. These results are similar to those of Casteangia et al. study, that they used combination of quercetin and curcumin31.
Quercetin is a hydrophobic substance and high percentage of its incorporation (Table 1) is related to its tendency to position in between the bilayer shell of liposomes rather than aqueous phase. In this study F3 was excluded from the rest of the study because of sedimentation of un-loaded drug after centrifuge that could be because of saturation of hydrophobic regions in liposomes.
The cumulative amount of quercetin released from each formulation was plotted as a function of time over 480 min as shown in Fig. 1. Our results agree with the general conclusion that liposomal entrapment of drugs sustains their release. These results agree with the results of D. Liu et al. study. In his study, he compared the release of quercetin from solution and liposomal formulation, and finally concluded that liposomal entrapment of drugs sustains their release.32
Stability test also showed reasonable results regarding size and encapsulation efficacy of F1 and F2 during 3 month storage.
CONCLUSION
Fusion technique is used as a method for making liposomal forms in this study and resulted in preparations with enough viscosity for topical application. The particle size of the entire formulation is smaller than 60 nm. Liposomes showed high incorporation efficiency which may due to lipophilic nature of quercetin. Formulation F2 exhibited the highest drug release at 480 min and no significant changes were observed regarding particle size and incorporation efficiency during 3-month storage at 4 °C. These results suggested that optimum formulation could gradually release quercetin and decrease adverse effects and also increased patient compliance. Quercetin has anti-oxidant and anti-inflammatory properties, but as it is mentioned in another studies, topical administration of quercetin in conventional dosage forms would not be suitable regarding anti-oxidant and anti-inflammatory effects whereas using lipid vehicles is the most suitable way to deliver and increase the retention of quercetin in the skin by weaken the barrier function of stratum corneum and facilitate drug permeation .
The overall results obtained in this study showed that liposomes are interesting carriers of quercetin and they could be an appropriate candidate for topical use and further studies examining the efficacy of formulations on pressure ulcer is in progress.