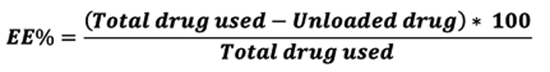Highlights
Deferoxamine can be topically used to improve diabetic ulcer healing due to its capability of iron-chelating and improving angiogenesis. Solid lipid nanoparticles as a novel drug delivery carrier was applied not only to protect the drug from environmental damaging factors, but also to provide a local high concentration and a sustained-release of the drug. In such a way the limitations of oral and injection routes of administration the drug will be eliminated.
Introduction
Diabetic foot ulcer is one of the most common problems occurred in diabetic patients, leading to significant disability and reduced quality of life such as amputation. Various factors are involved in the pathogenesis and occurrence of diabetic foot ulcers.1 Decreased neovascularization caused by impaired activity of the transcription factor hypoxia inducible factor-1 alpha (HIF-1α) is a major factor. Based on the pathogenesis, new methods are needed to effectively manage and treat diabetic wounds. Deferoxamine mesylate [DFO], a strong iron-chelating agent, increases HIF-1α transactivation by preventing iron-catalyzed reactive oxygen stress2, so it can be used to improve ulcer healing in diabetic people. It is also one of the rare medicines available at clinic used in iron poisoning to remove excess iron from the body and in thalassemia patients associated with the problem of iron cumulated in the body. Less than 15% of the drug is absorbed through the gastrointestinal tract, so it is used via injection.3,4
Unfortunately, DFO has two main problems limiting its clinical application in many cases. The first problem is the short plasma half-life (below 90 minutes) that makes it inevitable to be applied in repeated doses or in the form of continuous and long-time injection. Secondly, it may cause acute or chronic poisoning that the latter is more likely to occur due to the necessity of long-term application of the drug in thalassemia and similar patients.5
On the other hand, its oral dosage form has been studied in which some gastrointestinal side effects such as nausea, vomiting, and abdominal pains have occurred in one third of subjects. Skin rash is also one of the side effects in both injection and oral administrations.6,7 Acute renal failure is the most important considerable side effect of this medicine. In addition, it can be used in diabetic foot ulcer since it is discovered that due to the raise of glucose inside cells, activation of destroying mechanisms, like the production of free radicals, takes place. On this occasion, iron ion as a cofactor, has an important role in the conversion of superoxide free radical to poisonous hydroxyl radical, so avoids the continuation of destructive reactions and improves angiogenesis.8,9
Taking into account instances mentioned above, seems that by providing and applying topical DFO loaded in solid lipid nanoparticles [SLN], we can produce a high concentration of it on diabetic ulcers in order to both eliminate injection problems and avoid systemic side effects of the drug. Considering the role of skin lipids in the barrier system, lipid-based carriers such as solid lipid nanoparticles can exchange the skin lipid resulting in improving dermal penetration.10
Solid Lipid Nanoparticles, which were first mentioned in 1991, are colloidal lipid carriers, very much like nanoemulsions, but differing in lipid nature. SLNs are obtained from physiological-like lipids and surfactants which are GRAS (Generally Recognized As Safe), free of toxicity. SLNs have a number of advantages over traditional colloidal systems, such as physical stability, protection of the active substance against chemical degradation, controlled release of the active substance, biocompatibility, selective targeting, absence of organic solvents, and easy to scale up and sterilize.11-13 Solid lipid nanoparticles are attractive not only in the pharmaceutical industries but also in cosmetics and food industries.14-16
SLNs combine the advantages and avoid the disadvantages of other colloidal carrier systems such as liposomes and polymer nanoparticles. They could prolong the residence time of the dosage form at the absorption site, consequently increasing bioavailability. Controlled drug delivery, enhanced bioavailability of entrapped drugs and/or improved tissue distribution, good tolerability, and drug targeting have been attributed to SLN formulations.17
Due to such capability of solid lipid nanoparticles in long-term keeping of drug on injured skin and avoiding application of medicine in the form of injection or edible medicine and consequent prevention of attributed side effects, it was aimed to formulate DFO in the form of solid lipid nanoparticles and to study its physicochemical properties such as particle size and size distribution, entrapment efficiency, profile release, thermal behavior. The main objective of this study was to entrap DFO in solid lipid nanoparticles and investigate the characteristics of drug loading and release. Such particles, due to their nature, will remain for a long time on the applied area and will provide a slow-released and long action. Then, it is aimed to optimize the best formulation of the drug to provide conditions for studying on diabetic ulcers in future studies.
Materials and methods
Deferoxamine mesylate powder was purchased from Tolid Daru company(IR Iran), tween 80, PG, oleic acid, cholesterol, lecithin were obtained from Merck (Germany), span 20 were purchased from Pouyesh Kimia Gostar (IR Iran), cellulose acetate membrane was purchased from Tooba company (IR Iran), and compritol® 888 ATO (glyceryl behenate) was a gift from Gattefosse Pharmaceuticals Company (France). All chemicals and solvents were of analytical grade. Fresh double-distilled water was used in the experiments. Minitab16 software was used for experimental design and evaluation of the effect of variables on responses.
Assay of drug
For measurement, the drug loaded/released from formulations, a standard curve was plotted by preparing 6 solutions in different concentrations of the drug in phosphate buffer, pH=7.4, using a UV-visible spectrophotometer (CE250, CECIL, England) at 220 nm wavelength.
Experimental Design
To evaluate the effect of type of surfactant and type/amount of lipid, full factorial experimental design was applied. Based on this, 16 formulations in triplicate were prepared. The independent variables included the percentage of total lipid phase (%L), Liquid lipid to Solid lipid ratio (LL/SL), type of surfactant, and type of lipid.
Preparation of deferoxamine-loaded SLNs
SLNs were prepared using an emulsion-congealing technique by cold high-pressure homogenization. The lipid phase consisted of a liquid oil (oleic acid), a solid lipid (cholesterol or comperitol 888 ATO), and a surfactant (Tween 80 + Span 20,1:1, or lecithin); the aqueous phase composed of water and 50 mg DFO. The amounts of lipids and surfactants were varied according to factorial design presented in Table 1. The oil phase was melted at 65ºC, added to the aqueous phase at the same temperature and mixed for 3 minutes at speed of 12000 rpm using a high-speed homogenizer (Heidolph, Germany) to obtain a clear medium. Afterward, the mixture was further treated with sonication (90 W for 2 min); the emulsion was then diluted and congealed by adding a mixture of 4:1 water-propylene glycol to reach a final volume of 50 ml. The final concentration of DFO was 1mg/ml according to some studies. Finally, the suspension was passed through a high-pressure homogenizer (Emulsiflex-C3, Avestin, Canada) for 3 cycles (20 seconds for each cycle) at 1000-2000 bars.18-20
Table 1. Independent variables and their levels
| Variable | High level | Low level |
|---|---|---|
| Lipid | Cholesterol + oleic acid (90:10) | Comperitol + oleic acid (90:10) |
| %L | 90% | 80% |
| Surfactant | Tween 80 + Span 20 (1:1) | Lecithin |
| LL/SL | 20:80 | 10:90 |
%L: Percentage of total lipid phase, LL/SL: Liquid lipid to Solid lipid ratio
Measurement of particle size
Particle size analyzer instrument (Malvern, Master SIZER 2000) was used to evaluate particle size and size distribution. Samples were diluted with a determined double-distilled water.
Determination of drug entrapment efficiency [EE%]
To do this, 5 ml of each formulation was centrifuged at 12000 rpm for 10 minutes. After separation the particles, they were precisely rinsed with water, and then the amount of unloaded drug was measured in the watery part at 220 nm wavelength. To calculate the entrapment efficiency, the following equation was applied:
Study of release profile of drug
Static Franz Diffusion Cell System was used to determine the profile of release of entrapped drug in nanoparticles. To do this, a predetermined weight of each formulation was put in the donor part of the diffusion cell. Phosphate buffer, pH 7.4, was used as the recipient phase. The donor and the recipient units were separated with cellulose acetate membrane having a cut-off of 10 kDa. The temperature and speed of stirring the recipient phase were adjusted on 37±0.5 °C and 200 rpm, respectively. Sampling was carried out by withdrawal of 2 ml from the recipient phase at time intervals of 0.5, 1, 2, 3, 5, 6, 7, 8, 12, 24, 36, 48, and 72 hours after starting the procedure. After each sampling, 2 ml of phosphate buffer was immediately added into the recipient to remain the volume equable. The cumulative amount of released drug was plotted versus time to obtain the release profile.
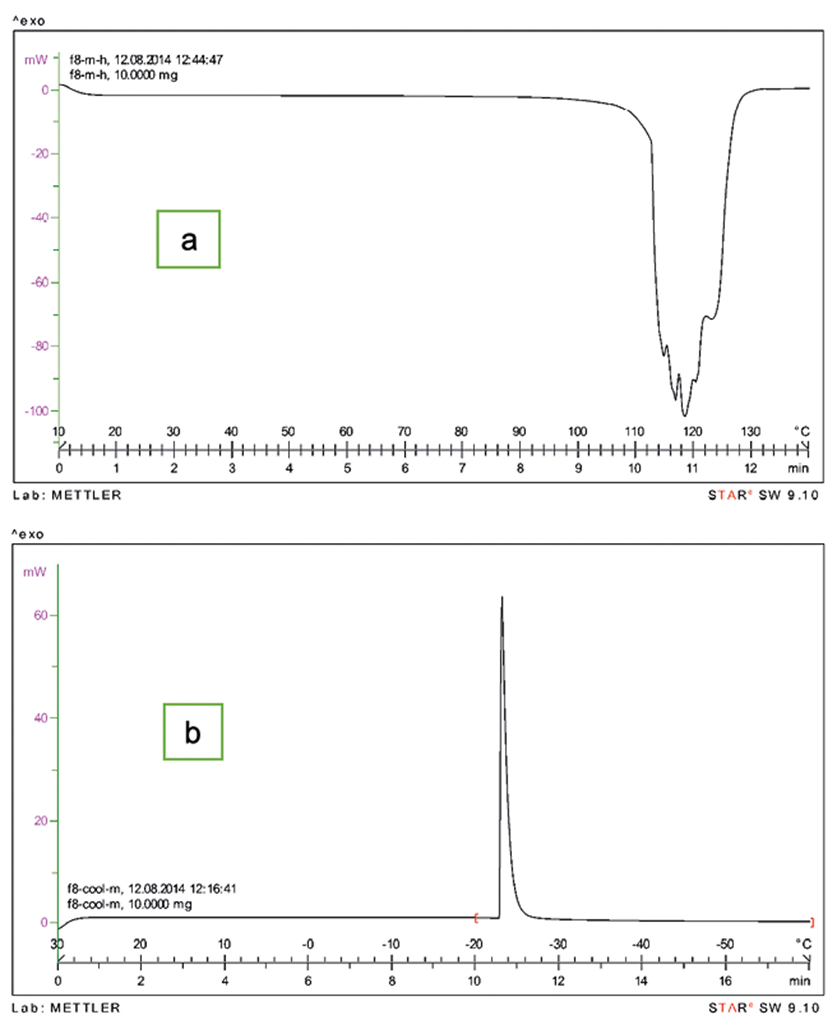
Thermal Behavior of Nanoparticles
To study the thermal behavior of nanoparticles, Differential Scanning Calorimetry (METTLER TOLIDO, Switzerland) was used through two profiles including heating (from +10 to +120° C) and cooling (from +30 to -60° C). Phase transitional temperature and enthalpy were determined and analyzed for each formulation.
Statistical Methods
Each formulation was prepared in triplicate, so the total numbers of formulations were 48. To analyze data, the Two-Way Analysis of Variance statistical test (ANOVA) was used to compare characteristics of formulations. In addition, for the study of the relationship between variables and responses, concurrent multiple regression was carried out. P-value equal to or less than 0.05 was considered as significant correlation in all experiments.
Results
Particle size
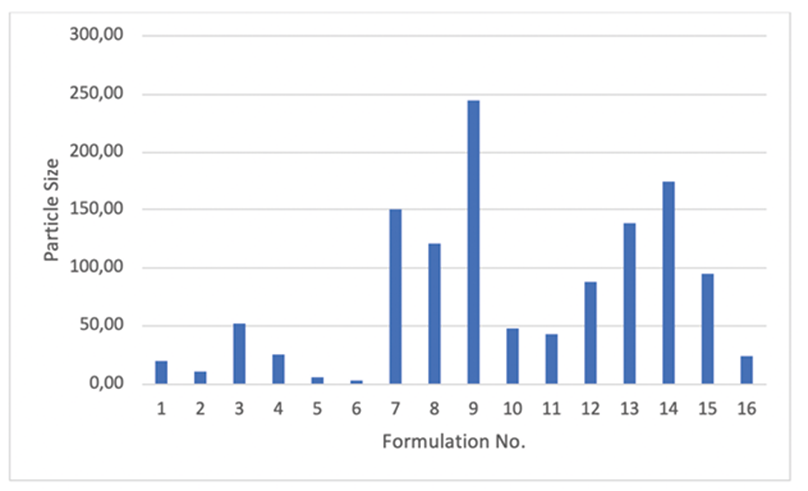
The particle size ranged from 2.88 to 174 nm for all formulations as shown in Figure 1.
The results show that most particles are less than 100 nm, so the method used to prepare formulations had been properly able to produce nanoparticles. However, it has been no significant correlation between variables and particle size. Furthermore, it was found the average of 0.344 for polydispersity index that indicates a narrow size distribution in all formulations regardless particle size.
Drug Entrapment Efficiency
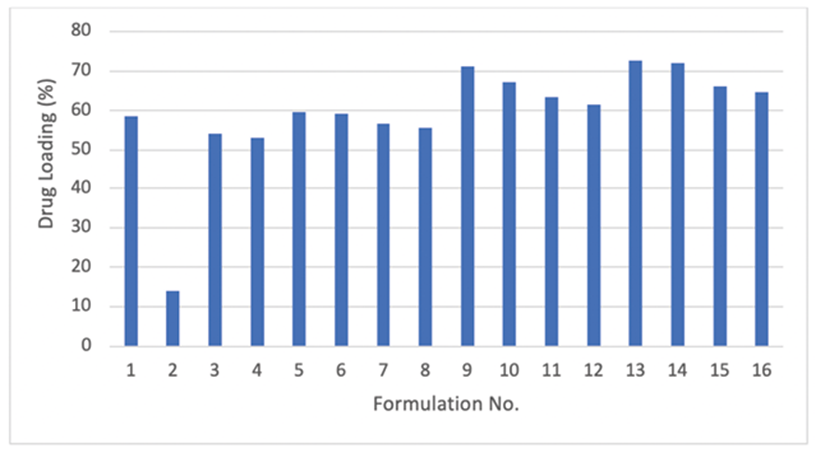
Determination of drug loaded in nanoparticles was assessed for all formulations. Figure 2 shows the drug loading percentage of SLN formulations.
As shown, except formulation No. 2, all other formulations had more than 53% of entrapment efficiency. This means that the contents of formulations were effective on the loading of drug. The average loading for all formulations was about 60% which is considerable for DFO as a hydrophilic drug. Analysis of regression between variables and %EE identifies a significant correlation between lipid content and drug loaded in particles (p=0.014). Changing the solid lipid from cholesterol to Compritol®, resulted in an increase in the loading percentage. Based on regression analysis, none of the other variables did significantly affect %EE (P>0.05). Compritol, Glyceryl behenate, a high melting point glyceride with a less-ordered crystal lattice, is appropriate for use in dosage forms as a lipid matrix to increase drug entrapment and to modify (sustain or delay) the release of active ingredients.21 It is safe and useful for increasing consistency and thickness for topical formulations. On the other hand, the lowest loading belongs to formulation No. 2 and the highest one belongs to No. 13. The latter includes Compritol and the higher level of LL/SL ratio which agrees with many of reports. According to these reports, the incorporation of liquid oil into solid lipid nanoparticles leads to increase crystal disorder and consequently results in great failure in the lattice and leaves more space to house the drug.22,23
Drug release profile
The drug release profile from SLNs is dependent on the preparation temperature, composition of emulsifier, and percentage of oil, especially liquid one, incorporated in the lipid matrix.23
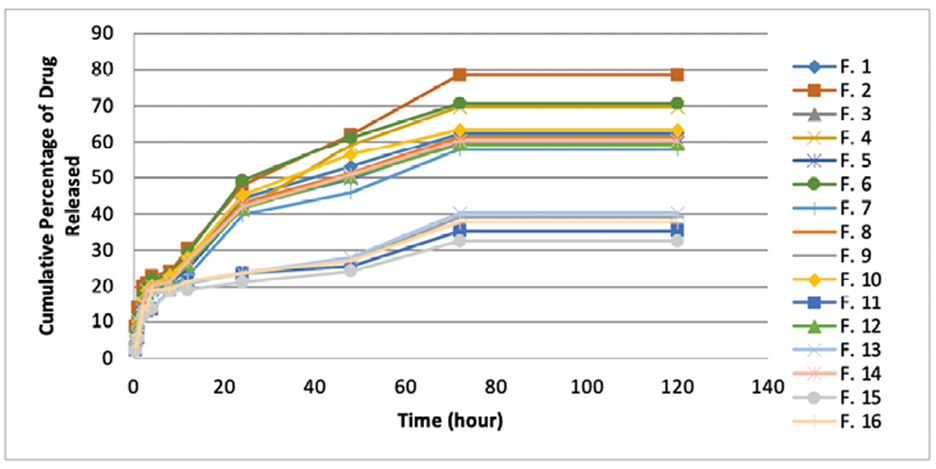
Since one of the objectives of this study was to provide a slow release formulation to prolong the effect, the two factors %R2h and %R72h were considered as indices for slow-release and sustained-release. The in vitro drug release profiles of SLN formulations consisted of an initial burst release followed by a sustained and slow release. The averages release after 2 and 72 hours were 15.06% and 55.54%, respectively.
Analysis of regression showed that both indices %R2h and %R72h had a significant direct correlation with %L, lipid content, and surfactant type (P=0.001), but a significant indirect correlation with LL/SL (P=0.001). Except for one, for all other formulations the average of %R2h was 20 percent and some of the formulations had released more than 60 percent of loaded drug after 48 and consequently 72 hours. Such profile is appropriate for a formulation which had been purposed for prolonged drug delivery. Besides, considering the initial burst release and followed by sustained release from formulations, it could be said that the drug is incorporated homogeneously, partially loaded in shell, interfacial and lipid core. This is in accordance with Ramteke K.H et al which reported the drug was homogeneously loaded in particles using cold homogenization technique.18
Figure 3 represents the cumulative percentage of drug released from all formulations after 2, 8, 48, and 72 hours, graphically.
Differential Scanning Calorimetery
DSC gives data from thermal behavior including melting and recrystallization of the solid lipids in SLNs. Various lipids have different melting points and enthalpies. The crystallinity degree of nanoparticles decreases with increasing liquid lipid ratio in the particles.23 The liquid oil existing in lipid matrix is the main factor lowering the crystallinity and increasing the less-ordered structure of SLNs.
Table 2 presents the DSC results for SLN formulations through both heating and cooling manners.
Table 2. DSC results of SLN formulations
| Formulation | ΔH | Phase Transitional Temp. | ||
|---|---|---|---|---|
| No. | Cooling | Heating | Cooling | Heating |
| 1 | 115.4 | -471 | -25 | 114 |
| 2 | 80.9 | -709.3 | -29 | 101 |
| 3 | 216.3 | -132.72 | -23 | 90 |
| 4 | 46.1 | -584.2 | -31 | 117 |
| 5 | 30.148 | -224.1 | -35 | 114 |
| 6 | 442 | -425.28 | -15 | 123 |
| 7 | 85.18 | -872.2 | -24 | 118 |
| 8 | 65.8 | -616.07 | -22 | 115 |
| 9 | 90.6 | -656.8 | -23 | 110 |
| 10 | 32.8 | -264.78 | -29 | 119 |
| 11 | 44.5 | -578.23 | -26 | 70 |
| 12 | 58.83 | -580 | -25 | 83 |
| 13 | 56.89 | -420.7 | -29 | 68 |
| 14 | 87.7 | -451.1 | -24 | 90 |
| 15 | 72.23 | -541.13 | -29 | 72 |
| 16 | 64.23 | -870.4 | -32 | 110 |
As shown in heating program, the phase transitional temperature of 68 °C, the lowest one, belongs to formulation No. 13 containing cholesterol and lower level of LL/SL ratio. The highest one, 123 °C, belongs to No. 6 which consisted of Compritol and the higher level of LL/SL ratio. This result is compatible with loading results. According to Chia-Lang Fang and et al, the decline of enthalpy and reduction of the melting point of the lipids occur in the SLNs with smaller size, higher surface area and a greater number of emulsifiers.23 Figure 4 represents 2 samples of DSC thermograms for formulation No. 8.
Discussion
The present study was designed to prepare a topical formulation of deferoxamine based on solid lipid nanoparticles. Such formulation can be used on diabetic foot ulcers to improve angiogenesis by removing iron ions and hence avoid the side effects of oral or repeated injectable deferoxamine as well as overcome the problem of short plasma half-life and poor bioavailability of the drug. Selection of SLNs for drug delivery was based on their ability to protect the drug from destructive environmental factors and provide sustained-release condition. Despite numerous benefits of SLNs, one of their disadvantages may be the low loading capacity due to the crystalline structure of solid lipids. The use of oleic acid, as results showed, overcame the drawback because of its less-ordered structure. Comperitol, compared to conventional solid lipids, has a higher melting point so that formulations containing Comperitol released the drug in a sustained manner. According to many reports, the minimized particle size and narrow size distribution can be obtained by applying high pressure homogenization technique.14,22 The particle size in the majority of formulations prepared at the present study was accordance with the reports. On the other hand, the application of cold homogenization technique was according to Rainer et al study in which the cold method was suggested for hydrophilic drugs to avoid partitioning the drug between the melted lipid and water phase during the hot homogenization. Relative high loading of deferoxamine in all formulations confirmed the suggestion. During cold homogenization method propylene glycol helps particles be spherical so provide the highest surface areas required for better loading and release of drug. It also increases loading capacity by preventing the loss of hydrophilic drug due to partitioning the drug into the water phase.12
Conclusion
deferoxamine mesylate, as a strong chelating agent, is used to remove excess iron from the body in poisoning cases and thalassemia patients. Since it must be injected repeatedly, so its adverse effects are unavoidable. There are some reports in which the beneficial effects of this drug on diabetic ulcers have introduced when applied locally. On the other hand, administration the drug using conventional dosage forms cannot provide a slow-release condition to avoid repeated use. According to the purpose of the study and considering all the results obtained for different formulations, formulation No. 13, which has a high level of drug loading and the lowest drug release rate, can be introduced as the best formulation. This formulation contained compritol and oleic acid in the amount of 8% of the total formula, as well as tween 80 and lecithin as a mixture of surfactants. This study showed that deferoxamine may be loaded in solid lipid nanoparticles to provide desired conditions. However, this requires evaluating drug-loaded SLNs in ex-vivo and in-vivo conditions to achieve other properties such as permeation capability through intact and ulcered skin.