Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
REC: Interventional Cardiology
versión On-line ISSN 2604-7276versión impresa ISSN 2604-7306
REC Interv Cardiol ES vol.5 no.1 Madrid ene./mar. 2023 Epub 18-Mar-2024
https://dx.doi.org/10.24875/recic.m22000340
ORIGINAL ARTICLES
High rate of uncovered struts in latest generation drug-eluting stents with durable, biodegradable polymer or lack of it 1 month after implantation
aServicio de Cardiología, Hospital del Mar, Barcelona, España
bDepartament de Medicina, Universitat Autònoma de Barcelona, Barcelona, España
cServicio de Cardiología, Hospital Universitario Bellvitge, Barcelona, España
dCentro de Investigaciones Biomédicas en Red de Enfermedades Cardiovasculares (CIBERCV), España
eInstitut Hospital del Mar d'Investigacions Mèdiques (IMIM), Barcelona, España
fFacultat de Medicina, Universitat de Vic – Universitat Central de Catalunya, Vic, Barcelona, España
gServicio de Cardiología, Hospital Son Espases, Palma de Mallorca, Mallorca, España
hServicio de Cardiología, Hospital Universitari I Politècnic La Fe, Valencia, España
iServicio de Cardiología, Hospital Universitario Marqués de Valdecilla, Instituto de Investigación Santiaria Valdecilla (IDIVAL), Santander, Cantabria, España
Abbreviations
DAPT: |
dual antiplatelet therapy. |
DES: |
drug-eluting stents. |
OCT: |
optical coherence tomography. |
PCI: |
percutaneous coronary intervention. |
QCA: |
quantitative coronary angiography. |
INTRODUCTION
Uncovered stent struts is one of the key predictors of stent thrombosis,1,2 and dual antiplatelet therapy (DAPT) has shown to reduce its risk.3 However, DAPT increases the risk of hemorrhage, and nearly one third of the patients treated with percutaneous coronary intervention (PCI) are considered at high bleeding risk. Given the desire for earlier discontinuation of DAPT to reduce the risk of bleeding complications, healing of the stents at earlier time points is desirable.
Drug-eluting stents (DES) significantly reduce neointimal hyperplasia and restenosis compared to bare-metal stents (BMS). However, the main concern regarding first-generation DES was late stent thrombosis due to lack of endothelization of stent struts.1 Therefore, a new generation of DES was developed based on improved thinner metal platforms, new drugs (alternative antiproliferative -limus analogues),4-6 and more biocompatible polymers.7 The evolution of DES moved towards DES with biodegradable polymers.8-10 The comparative studies between DES with biodegradable polymers and BMS showed a lower rate of cardiac death, target vessel-related myocardial reinfarction, and revascularization at 1 year.10 Compared to biodegradable polymer DES, those with durable polymer were noninferior regarding acute coronary syndromes with respect to all-cause mortality, nonfatal myocardial infarction, and revascularization.11 Furthermore, the most recent advance to overcome stent thrombosis has been the polymer-free DES. This type of DES was initially designed to reduce the risk of stent thrombosis in patients with high bleeding risk who could only take short courses of DAPT. It was compared to BMS showing a better efficacy and safety profile.13 It has recently been compared to DES in large clinical trials, especially in patients with high bleeding risk and need for shorter DAPT courses. In these studies, the use of polymer-based zotarolimus-eluting stent was noninferior to polymer-free DES14, and non-measurable differences in device-oriented composite primary endpoints were found.15
However, despite these large clinical trials, there is scarce information on the difference between the characteristics of arterial healing among different types of latest generation DES. Optical coherence tomography (OCT) is a widely used high-resolution intracoronary imaging modality to assess vascular response after stent implantation, thus detecting stent strut coverage and its apposition to the vessel wall.16,17 Stent strut coverage as studied by the OCT is considered a valuable surrogate marker for vessel healing after DES implantation.
The objective of this study was to compare durable polymer-coated everolimus-eluting stents (durable-polymer EES) vs biodegradable polymer-coated everolimus-eluting stents (biodegradable-polymer EES) vs polymer-free BES using stent strut coverage as assessed by the optical coherence tomography (OCT) as a surrogate marker to evaluate short-term arterial healing.
METHODS
Patient population and data collection
This was a prospective, multicenter, non-randomized study that compared 3 different types of DES: a) the durable polymer-coated everolimus-eluting stent Xience DES (Abbott, United States); b) the biodegradable polymer-coated everolimus-eluting stent Synergy DES (Boston Scientific, United States), and c) the polymer-free BES Biofreedom DES (Biosensors International Ltd, Singapore). The study was conducted at 4 Spanish teaching hospitals.
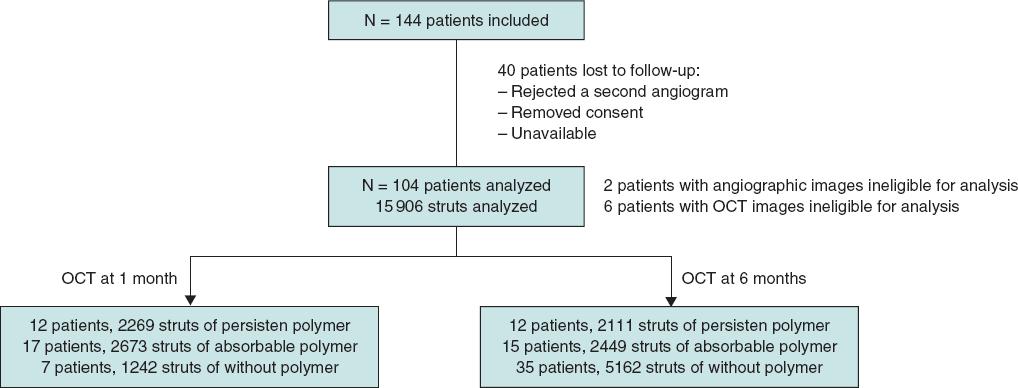
A total of 144 patients were consecutively recruited from January 2018 through December 2019. Patients were eligible if they had been admitted due to stable coronary artery disease or acute coronary syndrome without cardiogenic shock. The medical team selected the type of stent that should be implanted. The detailed study flowchart is shown on figure 1. Inclusion criteria were a) de novo lesions; b) ≥ 1 target lesions in the same or different coronary artery; c) no need for stent overlapping, and a 10 mm minimal distance between the stents; d) stent length between 8 mm and 30 mm; e) use of stents with diameters ≥ 2.5 mm.
Exclusion criteria were a) complex lesions including ostial lesions, chronic total coronary occlusions, calcified lesions requiring calcium modification techniques, and bifurcations requiring the kissing balloon technique; b) target lesions in small vessels (< 2.5 mm) and long lesions (> 30 mm) requiring small diameter stents (2.25 mm) or overlapping stents; c) diabetic patients; d) very tortuous arteries that anticipated the impossibility of access with the OCT catheter for follow-up purposes; and e) complications during index procedure. Patients with diabetes mellitus were excluded from the study because of their pro-inflammatory status that facilitates both stent thrombosis and restenosis.18
Once recruited, patients were consecutively assigned to a 1- or 6-month OCT follow-up group. The baseline characteristics, angiographic and procedural data, follow-up data, and outcome data were prospectively collected by the study coordinators. Clinical data at follow-up were obtained from the clinical records. This study was performed following the principles established by the Declaration of Helsinki, ISO14155, and the clinical practice guidelines. The study protocol was approved by the Institutional Ethics Committee (IEC) and the hospital research committee. Informed consent was obtained from all the patients.
Percutaneous coronary intervention, angiographic analysis, and optical coherence tomography
In the index procedure stents were implanted according to the standard approach. Patients were medically treated following the European guidelines on the management of chronic ischemic heart disease or acute coronary syndrome.19
Regarding the initial angiographic analysis, 2 orthogonal projections without coronary guidewire were obtained after finishing the index procedure. These same projections were acquired at follow-up. The off-line analysis of the angiographic images (quantitative coronary angiography [QCA]) was performed in an independent core lab (Barcelona Cardiac Imaging Core-Lab [BARCICORElab]), following their standard protocol. They used a dedicated software (CAAS, version 5.9; Pie Medical BV, The Netherlands). Methods used in this core lab have been previously reported20.
The follow-up angiography was performed at 1-or-6-month follow-up. Angiographic and OCT images were obtained from each patient. The Dragonfly frequency domain OCT C7-XR system (St. Jude Medical, United States) was used. This analysis was performed at the same independent core lab with a dedicated software (St. Jude Medical). Further information can be found on the supplementary data. The struts were classified as non-covered if their surface was totally or partially exposed to the lumen, and without any tissue coverage above its high-density scaffold. Stent strut apposition was defined as the perpendicular distance between the luminal edge of the strut and the vascular wall. Incomplete apposition was considered when distance was higher compared to the total strut thickness considering the addition of strut plus polymer. Intimal hyperplasia was measured as the perpendicular distance between the luminal surface of the stent strut and the luminal surface of the neointima.
Endpoints
The study primary endpoint was the percentage of uncovered struts among durable-polymer EES vs biodegradable-polymer EES vs polymer-free BES as seen on the OCT at 1 month.
The study secondary endpoint was to compare the coverage and apposition of these 3 different types of DES on the OCT 1 vs 6 months after implantation. In addition, we evaluated the intimal hyperplasia in the 3 stent groups over time.
Statistical analysis
Continuous variables were expressed as mean and standard deviation except when they did not follow normal distribution, in which case they were expressed as median and 25th-75th percentile. Categorical variables were expressed as frequency and percentage. The analysis of the clinical differences was performed using the chi-square test or Fisher's exact test for qualitative variables. Comparison among quantitative variables was performed using the 1-way ANOVA test. Generalized estimating equations, considering the clustering nature of the OCT data, were used to conduct analyses at strut level. All probability values were two-sided, P values < .05 were considered statistically significant. Statistical analysis was performed using the SPSS software package, version 22.0 (SPSS, United States). The sample size estimate is shown on the supplementary data.
RESULTS
Baseline clinical characteristics
A total of 104 patients from 4 different hospitals were included in the study; 44 patients were treated with a polymer-free BES, 35 with a biodegradable-polymer EES, and 25 patients with a durable-polymer EES. Of these, 37 patients underwent follow-up angiography and OCT 1 month after DES implantation, and 67 patients after 6 months. Mean age was 57 years; most patients were man (11% women). The inter-group baseline clinical characteristics are shown on table 1 according to the type of stent implanted. We observed a statistically significant difference in the left ventricular ejection fraction that was slightly lower in patients who received a polymer-free BES (54% vs 60%). The number of patients who needed postdilatation was higher in the durable-polymer EES group (68%) especially compared to the polymer-free BES group (38%).
Table 1. Baseline clinical, lesion, and procedural characteristics
| Polymer-free BES (N = 44) | Biodegradable-polymer EES (N = 35) | Durable-polymer EES (N = 25) | P | |
|---|---|---|---|---|
| Age | 57 ± 8 | 61 ± 9 | 59 ± 10 | .094 |
|
| ||||
| Women | 3 (7) | 4 (11) | 4 (16) | .482 |
|
| ||||
| Dyslipidemia | 24 (55) | 19 (54) | 14 (56) | .990 |
|
| ||||
| Hypertension | 17 (39) | 14 (40) | 13 (52) | .527 |
|
| ||||
| Family history of ischemic heart disease | 10 (23) | 4 (11) | 6 (24) | .353 |
|
| ||||
| Smoker | 26 (59) | 13 (37) | 9 (36) | .076 |
|
| ||||
| LVEF % | 54 ± 9 | 60 ± 9 | 60 ± 8 | .006 |
|
| ||||
| Chronic kidney disease (creatinine > 1.5 mg/dL) | 0 (0) | 1 (3) | 0 (0) | .370 |
|
| ||||
| Previous MI | 2 (5) | 7 (20) | 4 (12) | .102 |
|
| ||||
| Previous PCI | 2 (5) | 6 (17) | 4 (16) | .159 |
|
| ||||
| Previous CABG | 1 (2) | 0 (0) | 1 (4) | .526 |
|
| ||||
| Target lesion location | .101 | |||
|
| ||||
| Left anterior descending coronary artery | 18 (41) | 10 (29) | 11 (44) | |
|
| ||||
| Left circumflex artery | 10 (23) | 8 (23) | 19 (40) | |
|
| ||||
| Right coronary artery | 15 (34) | 13 (37) | 4 (16) | |
|
| ||||
| Secondary artery (diagonal, posterolateral, posterior descending) | 1 (2) | 4 (11) | 0 (0) | |
|
| ||||
| Stent length, mm | 18.6 ± 5 | 18.8 ± 6 | 19.5 ± 6 | .769 |
|
| ||||
| Stent diameter, mm | 3.4 ± 0.8 | 3.1 ± 0.5 | 3.1 ± 0.4 | .053 |
|
| ||||
| Predilatation, % | 19 (43) | 16 (73) | 13 (53) | .076 |
|
| ||||
| Postdilatation, % | 16 (38) | 12 (57) | 17 (68) | .05 |
Data are expressed as no. (%) or mean ± standard deviation. BES, biolimus-eluting stent; CABG, coronary artery bypass grafting; EES, everolimus-eluting stent; LVEF, left ventricle ejection fraction; MI, myocardial infarction; PCI, percutaneous intracoronary intervention; SD, standard deviation.
Procedural and lesion characteristics
Procedural characteristics based on the type of stent implanted are shown on table 1. We found no significant differences in stent diameter or length in the 3 stent groups. The left anterior descending coronary artery was the most treated of all whereas secondary arteries were scarcely included in this study.
Angiographic analysis
The lesion angiographic characteristics are shown on table 2 and table 1 of the supplementary data. There were 2 patients with angiographic images with insufficient quality for analysis, both from the biodegradable-polymer EES group. There were no significant differences in the lesions before or after the PCI. After 1 month, no significant differences were reported regarding lumen loss or percent diameter stenosis. No differences were reported among the 3 stent groups at 6 months.
Table 2. Angiographic analysis
| Polymer-free BES (N = 44) | Biodegradable-polymer EES (N = 35) | Durable-polymer EES (N = 25) | P | |
|---|---|---|---|---|
| 1-month follow-up | (N = 7) | (N = 16) | (N = 12) | |
|
| ||||
| Stent length, mm | 17.85 ± 4.32 | 19.24 ± 5.63 | 19.39 ± 4.41 | .788 |
|
| ||||
| Reference lumen diameter, mm | 2.93 ± 0.60 | 2.80 ± 0.53 | 2.77 ± 0.55 | .827 |
|
| ||||
| Minimal lumen diameter, mm | 2.75 ± 0.46 | 2.65 ± 0.50 | 2.51 ± 0.49 | .586 |
|
| ||||
| Late lumen loss, mm | 0.03 ± 0.09 | 0.04 ± 0.10 | 0.03 ± 0.08 | .965 |
|
| ||||
| Percentage diameter stenosis, % | 5.57 ± 6.27 | 6.50 ± 7.14 | 8.67 ± 9.27 | .658 |
|
| ||||
| 6-month follow-up | (n = 37) | (n = 17) | (n = 13) | |
|
| ||||
| Stent length, mm | 18.99 ± 4.92 | 20.03 ± 6.55 | 18.13 ± 4.95 | .627 |
|
| ||||
| Reference lumen diameter, mm | 2.75 ± 0.57 | 2.79 ± 0.50 | 2.65 ± 0.34 | .757 |
|
| ||||
| Minimal lumen diameter, mm | 2.54 ± 0.45 | 2.34 ± 0.41 | 2.39 ± 0.37 | .213 |
|
| ||||
| Late lumen loss, mm | 0.19 ± 0.25 | 0.28 ± 0.24 | 0.20 ± 0.18 | .368 |
|
| ||||
| Percentage diameter stenosis, % | 5.77 ± 15.30 | 15.18 ± 12.92 | 10.08 ± 7.40 | .065 |
Data are expressed as no. (%) or mean ± standard deviation. BES, biolimus-eluting stent; EES, everolimus-eluting stent.
OCT outcomes
OCT results are shown on table 3, and table 2 in the supplementary data. There were 6 patients with OCT images with insufficient quality for analysis, 2 from the polymer-free BES, 3 from the biodegradable-polymer EES group, and 1 from the durable-polymer EES group. Stent strut coverage and apposition were analyzed, as well as neointimal hyperplasia. Overall, 15 906 struts were examined. Of these, 4380 struts were from durable-polymer EES; 5122 from biodegradable-polymer EES; and 6404 from polymer-free BES; 6184 and 9722 struts were analyzed at 1 and 6 months, respectively.
Table 3. Optical coherence tomography analysis
| Polymer-free BES | Biodegradable-polymer EES | Durable-polymer EES | Pa | Pb | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Follow-up | 1-month (N = 7) | 6-months (N = 35) | P | 1-month (N = 17) | 6-months (N = 15) | P | 1-month (N = 12) | 6-months (N = 12) | P | ||
| Strut level analysis | (N = 1242) | (N = 5162) | (N = 2673) | (N = 2449) | (N = 2269) | (N = 2111) | |||||
|
| |||||||||||
| Uncovered struts, n | 238 (19.2) | 154 (3.0) | < .001 | 318 (11.9) | 123 (5.0) | .007 | 396 (17.5) | 133 (6.3) | .001 | .209 | .172 |
|
| |||||||||||
| Malapposed struts, n | 66 (5.3) | 13 (0.3) | < .001 | 101 (3.8) | 21(0.9) | .001 | 138 (6.1) | 35 (1.7) | .029 | .497 | .071 |
|
| |||||||||||
| Neointimal thickness, µm | 50.7 ± 41.9 | 138.1 ± 102.9 | < .001 | 59.9 ± 45.1 | 88.3 ± 83.9 | .005 | 48.9 ± 38.1 | 85.5 ± 68.6 | < .001 | .083 | < .001 |
Data are expressed as no. or mean ± standard deviation. Categorical variables were estimated using the chi-square test; quantitative variables were estimated with 1-way ANOVA test, and strut level analyses were conducted using generalized estimating equations considering the clustering nature of OCT data. BES, biolimus-eluting stent; EES, everolimus-eluting stent; ISA, incomplete stent apposition; RUTTS, ratio of uncovered to total stent struts.
acomparison at 1-month follow-up.
bcomparison at 6-month follow-up.
A high rate of uncovered struts was found among stents 1 month after implantation, with no significant differences (P = .209). We observed ≥ 5% of uncovered struts in > 80% of the patients. There was better coverage in the 3 stent groups at 6 months compared to 1 month (P < .001 polymer-free BES; P = .007 biodegradable-polymer EES; P = .001 durable-polymer EES). No statistically significant differences were reported in strut coverage at 6 months among the different stents (P = .172) (figure 2, figure 3A).
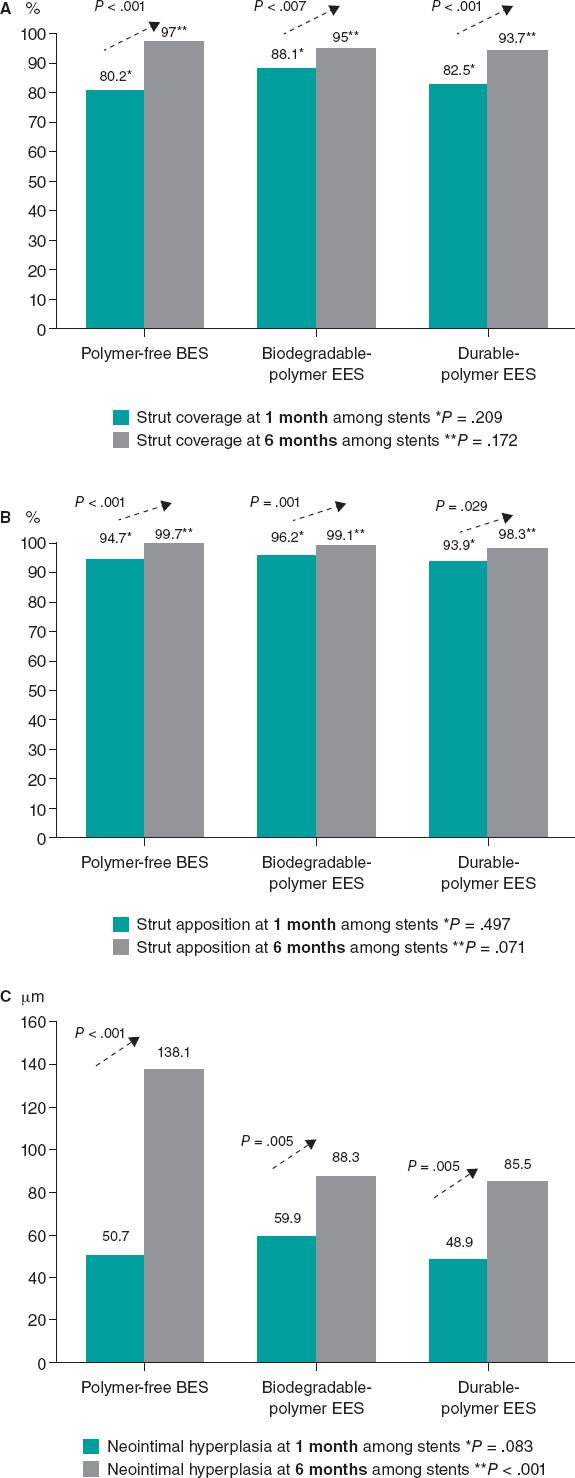
Figure 2. Endpoint comparison at 1 and 6 months. A: percentage of struts covered at 1 and 6 months; B: percentage of struts apposed at 1 and 6 months; C: neointimal hyperplasia at 1 and 6 months. BES, biolimus-eluting stent; EES, everolimus-eluting stent.
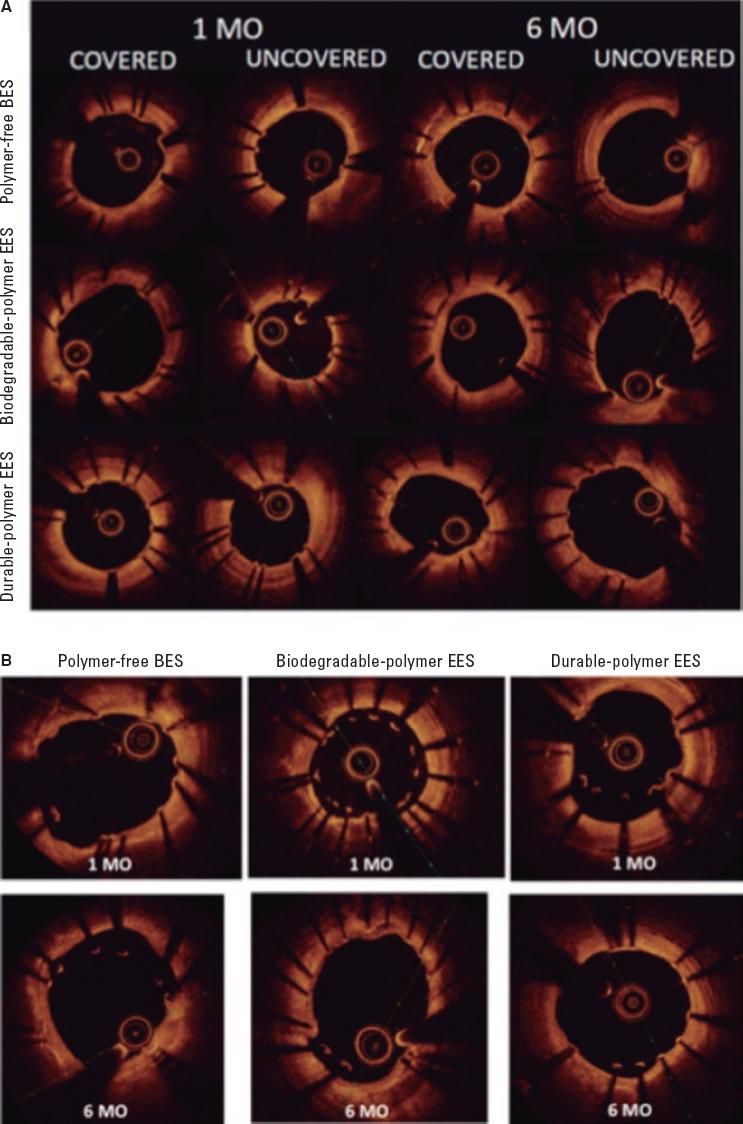
Figure 3. Optical coherence tomography. A: optical coherence tomography showing covered and uncovered stent struts at 1 and 6 months; B: optical coherence tomography showing malapposed stent struts at 1 and 6 months. BES, biolimus-eluting stent; EES, everolimus-eluting stent; MO, months.
Regarding strut apposition to the artery walls, no significant differences were reported among the 3 stents after 1 month (P = .497). We observed ≥ 5% of malapposed struts in 29% to 30% of the patients with no differences among stents. No significant differences were reported among the stents after 6 months either. The rate of apposition was higher at 6 months compared to 1 month in all stent groups (P < .001 polymer-free BES; P = .001 biodegradable-polymer EES; P = .029 durable-polymer EES) (figure 2, figure 3B).
When we analyzed neointimal hyperplasia, no significant differences were found at 1 month among the 3 stent groups (P = .083). At 6 months, we found higher hyperplasia in the polymer-free BES compared to the durable-polymer EES (P < .001). We found more hyperplasia at 6 months compared to 1 month in all groups (P < .001 polymer-free BES; P < .001 biodegradable-polymer EES; P = .005 durable-polymer EES; figure 2).
DISCUSSION
The main findings of this prospective multicenter registry are: a) in non-diabetic patients with de novo non-complex coronary lesions treated with durable vs biodegradable vs polymer-free DES, strut coverage was similar and low (≥ 5% of uncovered struts in > 80% of patients) at 1 month; b) there was a similar high rate of malapposed stent struts (4% to 6%) at 1 month; c) intimal coverage and apposition improved significantly at 6 months; d) polymer-free BES had higher intimal hyperplasia at 6 months.
OCT findings suggest that, in non-diabetic patients with non-complex coronary lesions treated with 3 latest generation DES, there is a similar high rate of uncovered and malapposed struts at 1 month. It is after 6 months when we could see better coverage and apposition.
Several small-scale OCT studies have been performed to compare the coverage and apposition of stents with permanent and absorbable polymers.21-24 Conclusions of these studies differ with most of them stating that the absorbable polymer stent has better coverage than the permanent polymer, and 1 of them concluding the opposite.23 One of the studies found coverage to be sufficient after 3 months,22 whereas another stated that coverage improved at 12 months.24 In this study, we found no significant differences in stent strut coverage or apposition between permanent and absorbable polymer at 1 or 6 months on the OCT analysis (figure 2. Differences in stent strut coverage at follow-up may in part be explained by the stent platform, the polymers used to control drug release, and the antiproliferative drug itself. The stents of the study had a similar drug (-limus analogue) but different polymeric features (durable vs biodegradable vs polymer-free), and different platform thickness (the polymer-free BES had a thicker platform). Probably within the first month the drug effect is most important, and it was similar among the study stents (antiproliferative -limus analogues). However, over time (between 1 and 6 months), other stent features such as platform thickness or polymeric features might play a role that could explain the differences seen regarding coverage at 3 months in other studies.22,23
Accordingly, other studies have analyzed other types of polymer-free stents different to the one from our study.25,26 They found that coverage was achieved in a high percentage at 3 to 9 months, reaching conclusions that were similar to our study. One of the studies26 performed an OCT at 1, 3, and 9 months, demonstrating higher strut coverage over time, which is consistent with our results. Only 1 study analyzed the Biolimus A9 polymer-free stent with OCT without comparing it to other stents.27 It was a prospective single-center single-armed study that examined strut coverage of the Biolimus A9 polymer-free stent at 1, 2, 3, 4, 5, and 9 months. Researchers found that coverage was fast and improved over time with the stent remaining safe and effective. These results are similar to ours in the sense that coverage was significantly better at 6 months. However, we also compared polymer-free stents to other polymer-based stents, something that, to the best of our knowledge, has not been tested before.
One of the limitations of extrapolating the clinical safety of the stents and the degree of intimal coverage as seen on the OCT is that there is no consensus on the cut-off value of coverage that would allow safe discontinuation of DAPT. Few studies1,28 have tried to decide on a percentage of coverage, with the only in-vivo study estimating that > 5.9% of uncovered struts was an independent risk factor for stent thrombosis.28 However, these studies were limited in the number of patients included, and only some stents were tested. Larger studies are needed to decide on a security threshold for strut coverage that would make it safe to stop DAPT without increasing the risk of stent thrombosis. Therefore, the rate of coverage at 1 month (80% to 88%) in our study seems insufficient, reaching a very high percentage after 6 months (94% to 97%) in the 3 types of stents.
Finally, our study shows that intimal hyperplasia was significantly higher in polymer-free stents at 6 months. Polymer-free Biolimus A9 has a stainless steel and thicker platform, which has been associated with more intimal hyperplasia and in-stent restenosis in previous studies.27 The other 2 types of DES have a cobalt-chromium platform which has largely substituted stainless steel to provide sufficient strength and visibility with thinner struts of around 70-90 μm, resulting in lower rates of angiographic and clinical restenosis.29 Thus, inflammatory response to this thicker stent platform (130-140 μm) could be in part responsible for this finding.
Study limitations
This was an OCT-based study; unfortunately, it was not powered to assess clinical outcomes. Our study was non-randomized. However, we minimized the confounding factors through selected inclusion/exclusion criteria for patients and lesions (non-diabetic patients with non-complex coronary lesions). We analyzed the differences among the groups and no significant differences were found, except in the left ventricular ejection fraction, which was significantly lower in the group of polymer-free stents. It has not been described in the medical literature whether a lower left ventricular ejection fraction has any correlation with stent thrombosis. This study included selected non-diabetic patients with simple coronary artery lesions. Therefore, the conclusions cannot be extrapolated to other groups with different characteristics.
The distribution of patients who underwent the follow-up angiography in the polymer-free DES group at 1 or 6 months was uneven. More patients rejected the follow-up angiography at 1 month in the polymer-free DES group, which may account for this difference. Finally, the complexity of the analysis of 3 different groups in 2 different moments of time caused a disgregation of cases. This led to a small N in each group, with the potential biases associated.
CONCLUSIONS
In non-diabetic patients, a significantly high percentage of uncovered struts was detected at 1 month with OCT in latest generation DES regardless of the polymeric features of the stent (durable vs biodegradable vs polymer-free stent) in the non-complex coronary artery lesion setting. Our OCT findings do not support improved short-term healing characteristics of stents with biodegradable polymer or polymer-free based -limus elution compared to current generation of durable polymer DES.
AUTHORS' CONTRIBUTIONS
A. Calvo-Fernández, R. Elosua, and B. Vaquerizo designed the study. J. Gómez-Lara, Héctor Cubero-Gallego, Helena Tizón-Marcos, N. Salvatella, A. Negrete, R. Millán, J.L. Díez, J.M. de la Torre Hernández, and B. Vaquerizo participated in the recruitment of patients and database contribution. A. Calvo-Fernández, C. Ivern, A. Sánchez-Carpintero, and B. Vaquerizo managed the database. A. Calvo-Fernández, X. Durán, N. Farré, and B. Vaquerizo conducted the data analysis and statistics. A. Calvo-Fernández, R. Elosua, N. Farré, H. Cubero-Gallego, and B. Vaquerizo drafted the paper with feedback from all the authors.
SUPPLEMENTARY DATA
Supplementary data associated with this article can be found in the online version available at https://doi.org/10.24875/RECICE.M22000343.
WHAT IS KNOWN ABOUT THE TOPIC?
Current DES with biodegradable polymer coating or the new polymer-free biolimus stent have been proposed as the optimal solution to the problem of delayed coronary artery healing characteristics seen with first-generation durable polymer-coated DES.
WHAT DOES THIS STUDY ADD?
This multicenter registry compared 3 types of DES with similar drug elution (-limus analogue), but different stent polymeric features (durable vs biodegradable vs polymer free).
Using the percentage of uncovered struts at short term assessed by optical coherence tomography as a surrogate endpoint, healing characteristics at 1 month were similar among stents and insufficient after 1 month.
Our findings do not support a preferential use of stents with biodegradable polymer-based or polymer-free stents to reduce the time of dual antiplatelet therapy at 1 month.
REFERENCES
1. Finn AV, Joner M, Nakazawa G, et al. Pathological Correlates of Late Drug-Eluting Stent Thrombosis:Strut Coverage as a Marker of Endothelization. Circulation. 2007;115:2435-2441. [ Links ]
2. Guagliumi G, Sirbu V, Musumeci G, et al. Examination of the In Vivo Mechanisms of Late Drug-Eluting Stent Thrombosis. J Am Coll Cardiol Intv. 2012;5:12-20. [ Links ]
3. Magnani G, Valgimigli M. Dual Antiplatelet Therapy After Drug-eluting Stent Implantation. Interv Cardiol. 2016;11:51-53. [ Links ]
4. Kelly CR, Teirstein PS, Meredith IT, et al. Long-Term Safety and Efficacy of Platinum Chromium Everolimus-Eluting Stents in Coronary Artery Disease:5-Year Results From the PLATINUM Trial. J Am Coll Cardiol Intv. 2017;10:2392-2400. [ Links ]
5. Maeng M, Tilsted HH, Jensen LO, et al. Differential clinical outcomes after 1 year versus 5 years in a randomised comparison of zotarolimus-eluting and sirolimus-eluting coronary stents (the SORT OUT III study):a multicentre, open-label, randomised superiority trial. Lancet. 2014;383:2047-2056. [ Links ]
6. Jakobsen L, Christiansen EH, Maeng M, et al. Final five-year outcomes after implantation of biodegradable polymer-coated biolimus-eluting stents versus durable polymer-coated sirolimus-eluting stents. EuroIntervention. 2017;13:1336-1344. [ Links ]
7. Sarno G, Lagerqyist B, Fröbert O, et al. Lower risk of stent thrombosis and restenosis with unrestricted use of 'new-generation'drug-eluting stents:a report from the nationwide Swedish Coronary Angiography and Angioplasty Registry (SCAAR). Eur Heart J. 2021;33:606-613. [ Links ]
8. von Birgelen C, Kok MM, van der Heijden LC, et al. Very thin strut biodegradable polymer everolimus-eluting and sirolimus-eluting stents versus durable polymer zotarolimus-eluting stents in allcomers with coronary artery disease (BIO-RESORT):a three-arm, randomised, non-inferiority trial. Lancet. 2016;388:2607-2617. [ Links ]
9. Raungaard B, Jensen LO, Tilsted HH, et al. Zotarolimus-eluting durable-polymer-coated stent versus a biolimus-eluting biodegradable-polymer-coated stent in unselected patients undergoing percutaneous coronary intervention (SORT OUT VI):a randomised non-inferiority trial. Lancet. 2015;385:1527-1535. [ Links ]
10. Jensen LO, Thayssen P, Maeng M, et al. Randomized Comparison of a Biodegradable Polymer Ultrathin Strut Sirolimus-Eluting Stent With a Biodegradable Polymer Biolimus-Eluting Stent in Patients Treated With Percutaneous Coronary Intervention. Circ Cardiovasc Interv. 2019;9:e003610. [ Links ]
11. Räber L, Kelbæk H, Ostojic M, et al. Effect of biolimus-eluting stents with biodegradable polymer vs bare-metal stents on cardiovascular events among patients with acute myocardial infarction:the COMFORTABLE AMI randomized trial. JAMA. 2012;308:777-787. [ Links ]
12. Kim HS, Kang J, Hwang D, et al, HOST-REDUCE-POLYTECH-ACS Trial Investigators. Durable Polymer Versus Biodegradable Polymer Drug-Eluting Stents After Percutaneous Coronary Intervention in Patients with Acute Coronary Syndrome:The HOST-REDUCE-POLYTECH-ACS Trial. Circulation. 2021;143:1081-1091. [ Links ]
13. Urban P, Meredith IT, Abizaid A, et al. Polymer-free Drug-Coated Coronary Stents in Patients at High Bleeding Risk. N Engl J Med. 2015;373:2038-2047. [ Links ]
14. Windecker S, Latib A, Kedhi E, et al;ONYX-ONE Investigators. Polymer-based or Polymer-free Stents in Patients at High Bleeding Risk. N Engl J Med. 2020;382:1208-1218. [ Links ]
15. Kufner S, Ernst M, Cassese S, et al;ISAR-TEST-5 Investigators. 10-Year Outcomes From a Randomized Trial of Polymer-Free Versus Durable Polymer Drug-Eluting Coronary Stents. J Am Coll Cardiol. 2020;76:146-158. [ Links ]
16. Lee KS, Lee JZ, Hsu CH, et al. Temporal Trends in Strut-Level Optical Coherence Tomography Evaluation of Coronary Stent Coverage:A Systematic Review and Meta-Analysis. Catheter Cardiovasc Interv. 2016;88:1083-1093. [ Links ]
17. Tearney GJ, Regar E, Akasaka T, et al. Consensus standards for acquisition, measurement, and reporting of intravascular optical coherence tomography studies:a report from the International Working Group for Intravascular Optical Coherence Tomography Standardization and Validation. J Am Coll Cardiol. 2012;59:1058-1072. [ Links ]
18. Yuan, J. Xu GM. Early and Late Stent Thrombosis in Patients with Versus Without Diabetes Mellitus Following Percutaneous Coronary Intervention with Drug-Eluting Stents:A Systematic Review and Meta-Analysis. Am J Cardiovasc Drugs. 2018;18:483-492. [ Links ]
19. Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019;40:87-165. [ Links ]
20. Gomez-Lara J, Teruel L, Homs S, et al. Lumen enlargement of the coronary segments located distal to chronic total occlusions successfully treated with drug-eluting stents at follow-up. EuroIntervention. 2014;9:1181-1188. [ Links ]
21. Teeuwen K, Spoormans E, Bennett J, et al. Optical coherence tomography findings:insights from the randomised multicentre trial investigating angiographic outcomes of hybrid sirolimus-eluting stents with biodegradable polymer compared with everolimus-eluting stents with durable polymer in chronic total occlusions(PRISON IV) trial. EuroIntervention. 2017;13:e522-e530. [ Links ]
22. Kretov E, Naryshkin I, Baystrukov V, et al. Three-months optical coherence tomography analysis of a biodegradable polymer, sirolimus-eluting stent. J Interv Cardiol. 2018;31:442-449. [ Links ]
23. Adriaenssens T, Ughi GJ, Dubois C, et al. STACCATO (Assessment of Stent sTrut Apposition and Coverage in Coronary ArTeries with Optical coherence tomography in patients with STEMI, NSTEMI and stable/unstable angina undergoing everolimus vs biolimus A9-eluting stent implantation):a randomised controlled trial. EuroIntervention. 2016;11:e1619-e1626. [ Links ]
24. Kim BK, Hong MK, Shin DH, et al. Optical coherence tomography analysis of strut coverage in biolimus- and sirolimus-eluting stents:3-Month and 12-month serial follow-up. Int J Cardiol. 2013;168:4617-4623. [ Links ]
25. Suwannasom P, Onuma Y, Benit E, et al. Evaluation of vascular healing of polymer-free sirolimus-eluting stents in native coronary artery stenosis:a serial follow-up at three and six months with optical coherence tomography imaging. EuroIntervention. 2016;12:e574-e583. [ Links ]
26. Worthley SG, Abizaid A, Kirtane AJ, et al. First-in-Human Evaluation of a Novel Polymer-Free Drug-Filled Stent. JACC Cardiovasc Interv. 2017;10:147-156. [ Links ]
27. Lee SWL, Tam FCC, Chan KKW, et al. Establishment of healing profile and neointimal transformation in the new polymer-free biolimus A9-coated coronary stent by longitudinal sequential optical coherence tomography assessments:the EGO-BIOFREEDOM study. EuroIntervention. 2018;14:780-788. [ Links ]
28. Won H, Shin DH, Kim BK, et al. Optical coherence tomography derived cut-off value of uncovered stent struts to predict adverse clinical outcomes after drug-eluting stent implantation. Int J Cardiovasc Imaging. 2013;29:1255-1263. [ Links ]
29. Pache J, Kastrati A, Mehilli J, et al. Intracoronary stenting and angiographic results:Strut thickness effect on restenosis outcome (ISAR-STEREO-2) trial. J Am Coll Cardiol. 2003;41:1283-1288. [ Links ]
Received: April 30, 2022; Accepted: September 28, 2022











 texto en
texto en