INTRODUCTION
Mammalian cells respond to electromagnetic field exposure by changing their proliferation rate and metabolism [1, 2]. Electrical currents via cell membranes are responsible for this response by activating intracellular synthetic pathways. The transmembrane currents, when generated by a flux of ions of calcium and phosphate, generate electromagnetic fields that produce a metabolic effect even at extremely low intensities of 0.02-0.03 mT [2, 3, 4]. This magnitude of the electromagnetic fields (EMF) is in the range of the background magnetic field on earth, i.e., 0.035-0.07 mT according to geographical location [5]. A higher EMF magnitude was found to be effective in the induction of pronounced cellular synthetic effects and caused a change in cell cycle [1]. This effect was found to be especially significant in cells of mesenchymal origin, and its application in an alternating mode enhances the maturation of these cells towards osteogenesis [4, 6, 7, 8, 9]. Naturally, this phenomenon has a highly significant clinical implication for the treatment of bone nonunion and for osseointegration into implants following orthopedic, maxillofacial and skull surgeries. Therefore, vast research efforts are being directed towards identifying optimal EMF parameters for enhancing osteoblast maturation, synthetic activity and proliferation. Alternating the EMF (AEMF) application was found to be especially effective for this purpose (Table 1) but the exact tendency pattern is still elusive. For example, the same maximal magnetic flux of 1.8 mT causes a decrease in cellular alkaline phosphatase (AP) (the early marker of osteoblast maturation) synthesis when applied in an alternating mode of 30 Hz [10], but has an opposite incremental effect when applied in a pulsed mode (PEMF) of 15 Hz with a basic 4 kHz frequency [7]. A higher intensity 5 mT AEMF of 15 Hz and 5 mT PEMF (basic frequency 50 kHz in pulses of 15 Hz) causes a similar incremental effect on cellular AP synthesis but with opposite cell proliferation responses when PEMF induces cell proliferation and AEMF reduces it [8, 9]. A higher intensity 6 mT PEMF also showed a significant incremental effect on cell proliferation [11]. It seems that this puzzling apparent lack of clear uniform tendency of cell response either to AEMF or PEMF with different frequency and magnetic flux paramenters might be explained by the suggested "window effect" theory of the nonlinear nature of modulation EMF frequency and intensity, which is related to the characteristics of calcium transmembrane flux [12]. The exact cellular mechanism of this penomenon is not known, but there is a clear indication that there are distinct and different ranges and combinations of AEMF and PEMF frequencies and intensities for affecting different cellular response of proliferation, metabolic activity and cell cycle modulation. Recognizing these combinations might provide powerful therapeutic tools for enhancing bone regeneration.
Table 1. Synopsis of the published data on the response of cultured osteoblasts to alternating electromagnetic field (AMEF) and pulsed electromagnetic field (PEMF)
↑: Significant increase; ↓: Significant decrease; mT: Millitesla; Hz: Hertz.
Previously published data show that even an extremely low 14.9 Hz PEMF intensity of 0.4 mT elicits a significant increase in cell maturation (indicated by cellular AP activity) and proliferation in cultured osteoblasts [6]. Since the control cultures for comparison are exposed only to a background earth magnetic field, which doesn't exceed 0.07 mT [5], the intriguing question is whether there is intensity lower than 0.4 EMF but higher than the background EMF that might affect human osteoblasts by enhancing proliferation and/or metabolic activity. An answer to this question might define the triggering values of EMF that induce cellular response.
Accordingly, we hypothesize that AEMF and/or PEMF having intensity lower than the currently known effective EMF for osteoblast stimulation for proliferation and maturation might still be effective. Therefore, the objective of this study was to investigate the response of human osteoblast-like cells in a monolayer culture to AEMF and PEMF with a maximal intensity of 0.2 mT, which is half the magnitude of the lowest reported stimulation values of EMF to date [6]. It should be noted that this EMF intensity is still a higher order of magnitude than the background magnetic field (0.035-0.07 mT). By verifying this hypothesis, we might be able to identify the narrower range of extremely applied extremely low magnitude alternating EMF that triggers osteoblast stimulation.
METHODS
CELL CULTURE PROTOCOL
Explant cultures of osteoblast-like cells (adherent to the plastic surface of culture plates) from human cancellous bone were investigated. Fresh bone samples were collected from distal femurs of human donors. These samples were of a disposable material from knee arthroplasties for the treatment of knee osteoarthritis. The use of disposable bone samples was approved by the institutional Ethical Committee, and all of the donors gave their written informed consent. Bone pieces of cancellous bone of 2-3 grams were incubated in DMEM with heat-inactivated fetal calf serum (10%), 2 mM L-glutamine, 100 mM ascorbate-2-phosphate, 10 nM dexamethasone, 150 m/ml streptomycin at 37oC, 50 U/ml penicillin and 20 mM HEPES buffer in a humidified atmospheric environment of 95% air with 5% CO2 (v:v ) for 20-30 days. It has been shown previously that these cells express osteoblast-like characteristics, such as polygonal multipolar morphology, positive Von Kossa staining, expression and activity of the enzyme alkaline phosphatase, synthesis of osteopontin, synthesis of a collagen-rich extracellular matrix with predominantly type I collagen and small amounts of type III and V collagen, and non-collagenous proteins (sialoprotein [BSP] and osteocalcin), demonstrating matrix mineralization in vitro and bone formation in vivo [1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15].
The cells were transferred into 24-well plates. Each well was seeded with 2×103 cells.
ELECTROMAGNETIC EXCITATION CONFIGURATION (FIGURE 1)
Plastic 24-well plates with cultured osteoblast-like cells were mounted on a stage above a tunable electromagnetic source (12×5 cm copper coil solenoid connected to a tunable pulse generator, designed and constructed by the author).
The spectra of the EMF frequencies applied to the cells were recorded digitally using an oscilloscope (sampling frequency: 20MHz); the maximal EMF values were recorded using a magnetometer (KOSHAVA 5, Wuntronic GmbH, Munich) with an axial probe (No.1099255-A, 0.2T=0.8V). Measurements were made by placing the probe directly on and perpendicular to the well plate's plastic bottom surface in wells containing liquid media (temperature of 37oC).
The background magnetic field in the experimental setup and placement of the control samples had a constant value of 0.003 mT.
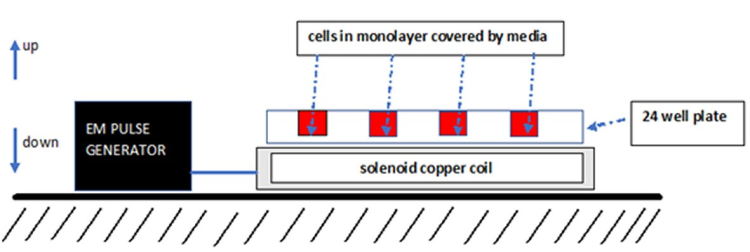
Figure 1. Simplistic schematic representation of the tunable experimental setup for the exposure of cultured osteoblast-like cells to an alternating pulsed electromagnetic field (AEMF) and pulsed electromagnetic field (PEMF).EM: Electromagnetic field.
The 10 kHz excitation protocol produced 85% of alternating EMF in the range of 9,999-10,000 Hz (Figure 2). The maximal EMF value was 0.2 mT.
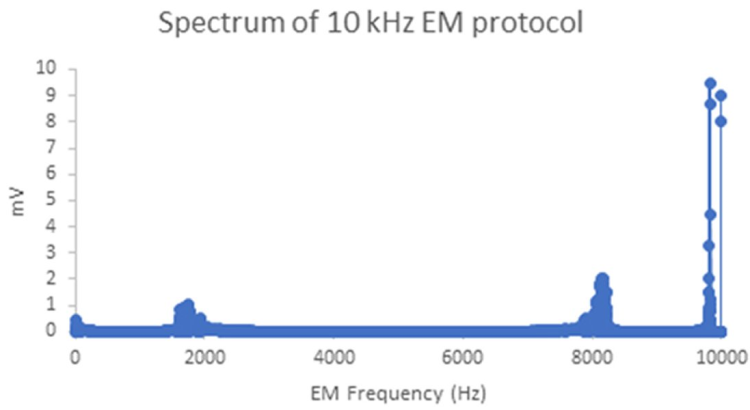
Figure 2. Spectrum of the alternating 10 kHz the electromagnetic field (EMF) excitation protocol (by Fast Fourier Transform of the EMF profile recorded on an oscilloscope).EM: Electromagnetic; Hz: Hertz.
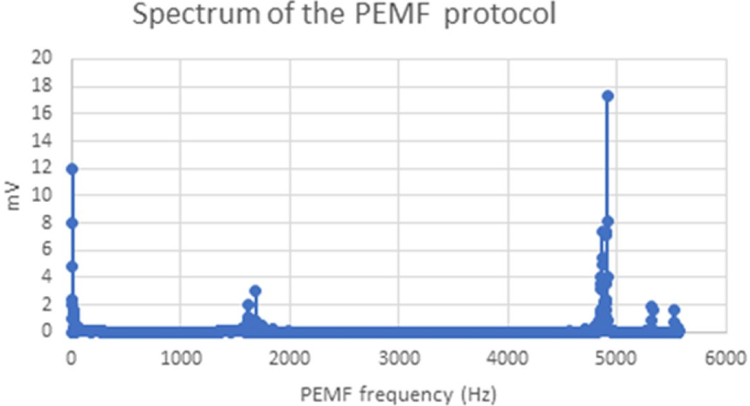
The PEMF excitation protocol produced 48% of alternating EMF in the range of 4,905-4,915 Hz (basic frequency of the EMF pulse) and 33% of 8-11 Hz (the EMF pulse rate) (Figure 3). The maximal EMF value was 0.2 mT.
EMF STIMULATION PROTOCOL OF THE CULTURED OSTEOBLAST-LIKE CELLS
Following the first passage of cells, replicates of an equal number of cultured cells were submitted to the excitation conditions of 10 kHz AEMF (Figure 2) or 10 Hz pulses with a basic 5 kHz frequency (PEMF, Figure 3). The duration and timing of exposure of the cultured replicates to the alternating EMF were chosen empirically according to previously found optimal protocols of mechanical stimulation of these cells by vibration [16, 17], i.e., four repetitions of two-minute exposure at 24-hour intervals. The cell stimulation was performed outside the incubator at room temperature, the control cultures kept in the same external conditions for the same period of time as the exposed to EMF cultures.
Doubling the time of the osteoblasts is dependent on seeding density. When seeded in the range of 103 cell/cm2 density (as was done in this study), the doubling time is around 10 hours [18]. Therefore, four days of the experiment (96 hours) represent 10 doubling periods. At this point, the cell culture doesn't reach 2D confluences when the proliferation rates cease, and the cells start with a higher metabolic activity in matrix elaboration. Therefore, during the four-day time period, the cells reach a maximal proliferation rate; accordingly, we evaluated the results of cell culture growth at this point in time.
We used the culture samples without exposure to the EMF protocol as controls. Six cell culture samples for each experimental condition were studied.
The number of cells in each culture sample was estimated by direct counting in a low-power microscopic field by marking the cells in each image using image processing software (ImageJ, NIH and the Laboratory for Optical and Computational Instrumentation, University of Wisconsin, USA). The average cell count up of three different microscopic fields for each culture sample was recorded. We chose this cell counting method to avoid interference with the culture conditions.
CELL PROLIFERATION AND CELL DEATH
Lactate dehydrogenase (LDH) specific activity, a marker of cell death due to LDH leakage via damaged cell membranes, was determined in culture media samples using 340 nm wavelength spectrophotometry of the reduced nicotinamide adenine dinucleotide (NAD), which is directly proportional to LDH activity [19, 20].
Cell proliferation was estimated as a product of the deduction of cell death change from the number of cells in the culture samples.
OSTEOBLAST MATURATION
Cellular AP activity, which is an early marker of osteoblast maturation from progenitors, increases in the Stage 2 osteoblast cell cycle after the Stage 1 cell proliferation ceases [21]. The cellular AP specific activity was measured using spectrophotometry [22]. Briefly, after counting the cells, the culture media from each sample were removed. Cells adherent to the plastic surface were washed by PBS and lysed in 0.1% Triton X-100 by three cycles of freezing at -20oC and thawing at 20oC. AP specific activity was measured in the lysed cell culture samples following incubation with P-nitrophenyl phosphate substrate by spectrophotometry (410 nm wavelength). For a comparison between the cultures conditions studied, the total values of AP specific activity in each culture sample were normalized to the number of cells in the microscopic field.
DATA ANALYSIS
One-way ANOVA and post-hoc Tukey tests were used to compare the results after the normal distribution of parameters had been verified. A p value less than 0.05 was considered as a significant difference between the compared values. Calculations of statistical comparisons were made using SigmaStat software (version 2, SPSS Inc., Chicago, Il, USA).
RESULTS
We found no difference in cell numbers in the culture samples exposed either to AEMF or PEMF compared to the unexposed controls (74±7SEM, 85±8SEM vs. 82±15SEM cells in the microscopic field, respectively, p>0.05, n=6, Figure 4).
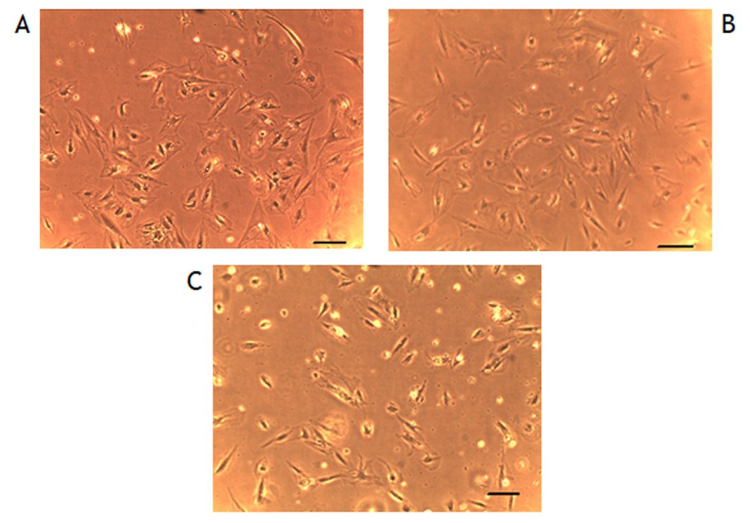
Figure 4. Representative examples of cultured cells' micrographs under different experimental conditions (scale 20 µm).A: Controls not exposed to external electromagnetic field (EMF). B: Cells exposed to 10 Hz EMF. C: Cells exposed to the pulsed electromagnetic field (PEMF). No gross difference in cell numbers is apparent in the experiment protocols.
There was no difference in LDH specific activity in the media of cultured samples exposed either to AEMF or PEMF compared to the unexposed controls (26.33±2.40SEM, 23.00±1.00SEM vs. 22.00±1.90SEM cells in the microscopic field, respectively, p>0.05, n=6).
The cellular AP specific activity increased significantly in the cell cultures exposed to the 10 kHz AEMF protocol compared to the unexposed controls (0.44±0.05SEM vs. 0.24±0.07SEM U/L/cells in the microscopic field respectively, p<0.05, n=6, Figure 5).
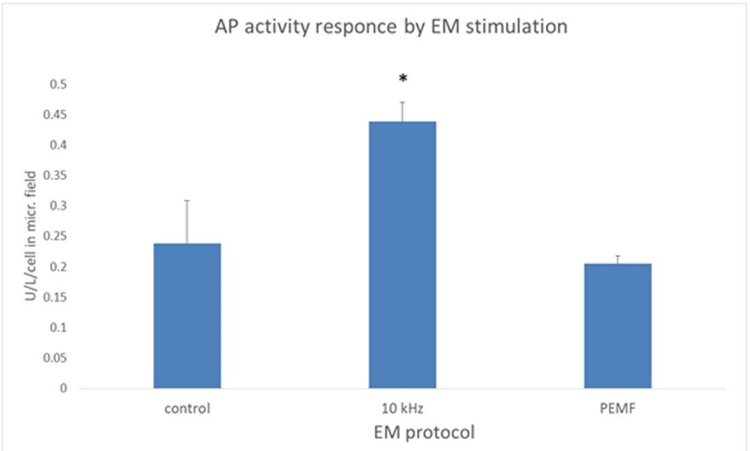
Figure 5. Alkaline phosphatase (AP) cellular activity is significantly increased following exposure to the 10 kHz alternating electromagnetic field AEMF protocol.Mean values with an indication of SEM (standard error of the mean), by the vertical bars, are presented. PEMF: Pulsed electromagnetic field - 10 Hz/5 kHz. EM: Electromagnetic; Hz: Hertz; kHz: Kilohertz; * p<0.05.
There was no significant difference in cellular AP specific activity in the cell cultures exposed to the PEMF protocol compared to the unexposed controls (0.21±0.02SEM vs. 0.24±0.07SEM U/L/cells in the microscopic field, respectively, p<0.05, n=6, Figure 5).
DISCUSSION
The exposure of cells to the 0.2 mT 10 kHz AEMF and 10 Hz/5 kHz PEMF had no significant effect on cultured human osteoblast-like cell death and proliferation. We can estimate the proliferation from the deduction of cell death values based on the number of cells in the monolayer culture. Since there was no significant difference in cell numbers and LDH specific activity in culture media (indication of cell death) between the culture samples exposed to PEMF and AEMF and the unexposed control culture samples, we can deduce that there is no difference in cell proliferation. Since the previous report showed that 14.9 Hz PEMF with maximal intensity of 0.4 mT induced cell proliferation [6], we can assume that the triggering values for osteoblast proliferation induction are in the range of 0.2 mT-0.4 mT ~10Hz range PEMF.
Contrary to the lack of proliferation modulation, the 10 kHz 0.2 mT AEMF induces a significant increase in AP cellular specific activity; therefore, the initial induction of osteoblast maturation can be induced by this extremely low AEMF. Importantly, this effect was not generated by the 0.2 mT PEMF. Accordingly, it is apparent that the triggering of osteoblast maturation can be induced by a lower AEMF intensity than that required for cell proliferation.
Therefore, according to our hypothesis, there is a triggering range for human osteoblast activation for maturation by an extremely low 10 kHz AEMF, at least 0.2 mT, which is distinct and below the triggering range of a PEMF for proliferation induction.
Thus, by recognizing these findings, the application of these EMF parameters in a clinical setup by a separate finetuning of osteoblast proliferation and maturation might have a therapeutic value in enhancing damaged bone regeneration.