INTRODUCTION
There has been an increase of patients with allergic conjunctivitis or similar allergic diseases, including allergic rhinitis and bronchial asthma, and these diseases can have a substantial negative impact on the quality of life (QOL) [1]. Allergic conjunctivitis is treated with oral anti-allergy agents/antihistamines or topical eye drops containing steroids/anti-allergy agents/antihistamines, but the response is often incomplete and symptoms persist [2].
Topical application of a drug can have effects on the deeper organs/tissues, e.g. application of a dermal patch preparation containing a nonsteroidal anti-inflammatory drug allows its direct penetration into the joint cavity to treat arthralgia [3, 4, 5, 6]. It was reported that topical application achieves higher drug concentrations in the treated subcutaneous tissue compared to the tissue concentration after oral administration [7, 8]. As the sites of inflammation in patients with allergic conjunctivitis (palpebral and bulbar conjunctiva) are located close to the palpebral skin, we considered that topical treatment with an ointment could be effective as supplemental therapy in patients with persistent symptoms despite current medications.
Therefore, we performed a pilot study to investigate the efficacy and safety of topical application of antihistamine ointment containing diphenhydramine (Restamin Cream®) to the eyelids in patients with allergic conjunctivitis.
METHODS
Among patients with bronchial asthma attending the outpatient department of National Hospital Organization Disaster Medical Center, Japan, those with allergic conjunctivitis due to pollinosis, etc. were enrolled in this study. The inclusion criteria were persistent symptoms despite treatment with anti-allergy agent/antihistamine eye drops, and no changes of the treatment regimen with anti-allergy agents, antihistamines, and steroids within 4 weeks before initiation of the study. Exclusion criteria were as follows: diseases of the eyelids such as atopic dermatitis, initiation of treatment with anti-allergy agents, antihistamines, or steroids within 4 weeks before the study, pregnancy/breastfeeding, and patients whom the attending physician considered inappropriate for other reasons.
The total duration of the study was 6 weeks, comprising a 2-week observation period before initiation of the study, a 2-week treatment period, and a subsequent 2-week washout period. During the treatment period, Restamin Cream® was applied to both eyelids twice a day at a dose of 0.1 FTU per eyelid (approximately 0.2 mg of diphenhydramine per eyelid each time; total daily dose of 0.8 mg). Patients received instructions about the application method from a pharmacist to ensure that the drug was not directly applied to the bulbar conjunctiva. During the entire study period, patients recorded their symptoms (eye itching and watering) on a visual analogue scale (VAS). In addition, QOL was assessed by using the Japanese Allergic Conjunctival Disease Standard QOL questionnaire (JACQLQ) Ver. 1 [9]. The JACQLQ contains 17 items from 6 domains (daily life, outdoor life, social life, sleep, body, and mental life) that are each scored from 0 to 4 points (0: None, 1: Mild, 2: Moderate, 3: Severe, 4: Very severe), after which the total score is calculated. QOL was assessed at 4 time points, which were at the start of the observation period, at initiation of treatment, at completion of treatment, and at the end of the washout period.
At the same 4 time points, anterior ocular findings were scored from 0 to 3 at the Department of Ophthalmology, and the total score was calculated for each of the right and left eyes. The following findings were assessed: conjunctival hyperemia, conjunctival congestion, palpebral edema, and changes of the palpebral conjunctiva (congestion, swelling, follicles, papillae, giant papillae), bulbar conjunctiva (congestion, edema), limbus (Trantas spots, swelling), and cornea (epithelium). In addition, visual acuity and intraocular pressure were measured at 3 time points, which were at initiation of treatment, at completion of treatment, and at the end of the washout period. The primary efficacy endpoint was VAS-based assessment by the patients, while the secondary endpoints were adverse reactions and changes of clinical findings.
Comparison of VAS scores among the different times of assessment, and comparison of ophthalmological findings or QOL between before and after each study phase was done with the Wilcoxon signed rank test.
This study was approved by the Ethics Committee of the National Hospital Organization Disaster Medical Center (approval number: 2016-22), and written informed consent was obtained from all of the subjects. This study was registered with the University Hospital Medical Information Network (UMIN) (Registry number: UMIN000026105).
RESULTS
PATIENTS
7 patients were enrolled in this study, but 2 patients discontinued treatment due to adverse reactions (Cases 6 and 7, Table 1). Prior treatment was olopatadine eye drops in 6 patients and ketotifen eye drops in 1 patient. Three patients were also using oral antihistamines, while 4 patients were not due to lack of efficacy (n= 1), drowsiness (n=1), and not wanting drug therapy (n=2). Inhaled steroids and long-acting β2-stimulants were used by all patients for their asthma. The severity of asthma ranged from step 2 (mild persistent asthma) to 5 (most severe asthma) according to the GINA (Global Initiative for Asthma) guidelines. Case 3 was on oral prednisolone (5 mg/day) for eosinophilic granulomatosis with polyangiitis.
EFFECT ON SYMPTOMS
All patients considered that application of the cream was effective for eye itching, including the 2 patients who discontinued the study. In addition, it was considered to be effective for eye watering by 6 patients, excluding Case 3. Except in Case 4, the onset of efficacy was relatively rapid and the duration of action was longer than that of eye drops. In all 5 patients who completed the study, symptoms of pollinosis tended to improve during the washout period because the pollen season ended.
VAS AND QOL SCORES
AS scores for itching of the eyes are shown in Table 2. Significant improvement of the VAS score was noted in 3 patients (Cases 2, 4, and 5). While 1 of these patients (Case 5) showed worsening of the VAS score after completion of treatment, the score improved further in another patient (Case 4). VAS scores for watering of the eyes are displayed in Table 3. Significant improvement of the VAS score occurred during treatment in 2 patients (Cases 2 and 5), although both patients showed worsening of the VAS score after completing treatment. On the other hand, the VAS score improved in 1 patient (Case 4) after completion of treatment. Case 1 stated that symptoms were considerably improved, but VAS scores for eye itching and watering did not decrease or actually increased. This patient subsequently reported that she had mistakenly recorded the symptoms she assumed would have occurred without topical therapy.
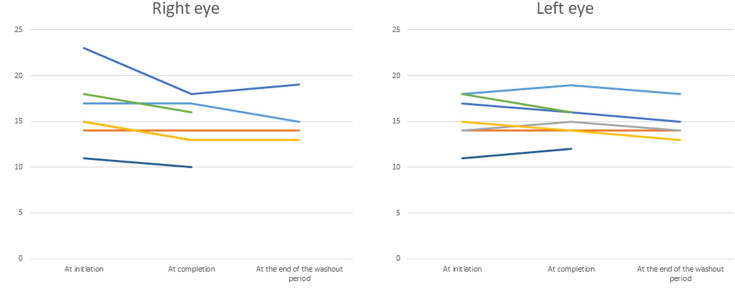
Based on the scores for anterior ocular findings, improvement of clinical findings was observed after treatment with the cream (p=0.0625, Wilcoxon signed rank test) and there was no obvious exacerbation during the subsequent washout period (Figure 1). QOL scores were also improved by application of the cream (p=0.0625, Wilcoxon signed rank test), with no exacerbation during the washout period (Figure 2).
Table 2. Visual analogue scale scores for eye itching
Significant improvement of the VAS score for itching was observed in 3 patients. In Case 1, marked improvement of symptoms was obtained, but the VAS scores did not correspond. The patient reported that she had mistakenly recorded the symptoms she assumed would have occurred without topical treatment.
Table 3. Visual analogue scale scores for eye watering
Significant improvement of the VAS score for eye watering was observed in 2 patients. In Case 1, marked improvement of symptoms was obtained, but the VAS scores did not correspond. The patient reported that she had mistakenly recorded the symptoms she assumed would have occurred without topical treatment.
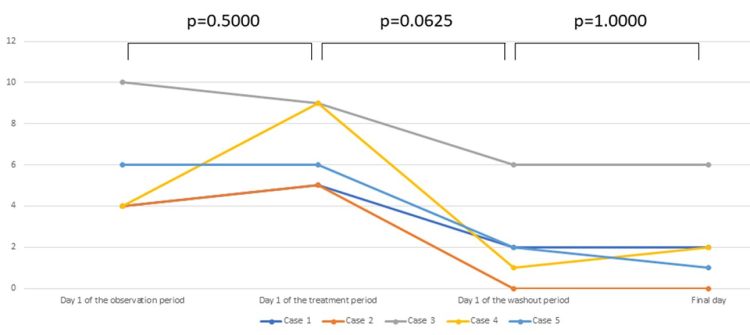
Figure 1. Clinical findings. Scores from 0 to 3 were assigned for ophthalmological findings of the palpebral conjunctiva (congestion, swelling, follicles, papillae, giant papillae), bulbar conjunctiva (congestion, edema), limbus (Trantas spots, swelling), and cornea (epithelium), and the total score was calculated for each of the right and left eyes.Improvement of clinical findings was observed after treatment (p=0.0625, Wilcoxon signed rank test). There was no exacerbation of findings in the washout period.
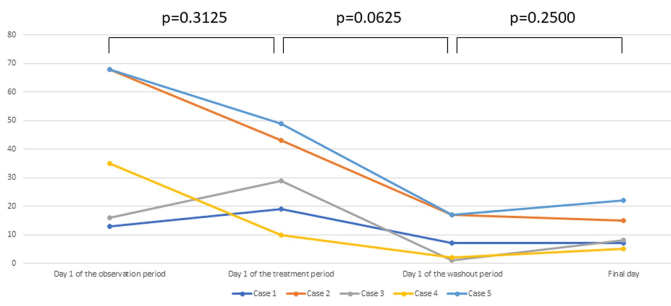
Figure 2. Assessment of QOL by the JACQLQ. Seventeen items from 6 domains (daily life, outdoor life, social life, sleep, body, and mental life) were scored from 0 to 4, and the total score was calculated. (0: None, 1: Mild, 2: Moderate, 3: Severe, 4: Very severe).Improvement of the QOL scores was observed after treatment (p=0.0625, Wilcoxon signed rank test). There was no deterioration of QOL after discontinuation of treatment.
SAFETY OUTCOMES
Adverse reactions occurred in 4 out of 7 patients (Table 1). Symptoms of allergic conjunctivitis showed marked improvement in the 2 patients who discontinued the study due to adverse reactions. One of these patients developed palpebral edema/pain and the other developed ocular erythema/pruritus associated with pain. These adverse reactions resolved spontaneously after discontinuation of treatment. Another 2 patients continued treatment despite adverse reactions: 1 patient had blurred vision during the first week of treatment and the other had mild tingling of the eyelids. There was no increase of intraocular pressure and no changes of visual acuity during the study in any of the patients, including the 2 who discontinued treatment (Figure 3).
DISCUSSION
This study was the first investigation of topical application of an antihistamine cream as treatment for allergic conjunctivitis and our findings suggest the usefulness of this method. Except in 1 patient, rapid control of symptoms was noted after application of the cream and its duration of action was longer than that of eye drops. Although all patients noted subjective improvement of their symptoms, local adverse reactions occurred at a high frequency. An over-the-counter eye drop medication (Stonarhini®) contains 4.5 mg of diphenhydramine per 15 mL, with the daily dosage being 0.045 mg to 0.27 mg per eye. The daily dosage of diphenhydramine in this study was 0.4 mg per eye, which was approximately 1.5 times higher than the maximum daily dose when using Stonarhini®. Local adverse reactions may have occurred at a high frequency due to administration of a high dose. Although the product is not directly applied to the conjunctiva, unlike eye drop medications, the optimal dose should be determined.
As topical therapy for ophthalmic disease, Vaseline labelled with fluorescein containing calcium carbonate has been applied to the eyelids to treat dry eyes [10]. The maximum lacrimal fluid concentration of fluorescein in the ointment was noted at 30 minutes after application, followed by a decrease to 20% of the peak level after 3 hours and little subsequent change after 6 hours. The severity of corneal epithelial disorder was unchanged after 3 months in the control group (Vaseline alone), whereas there was significant improvement in the study drug group. No adverse reactions occurred in either group. It is known that eyelid skin shows higher permeability than abdominal skin in rats (6 times higher for diclofenac and 11 times higher for tranilast), with the local drug concentration at 8 hours after application to the eyelids being higher than with eye drops [11]. Similarly, an effective conjunctival drug concentration was obtained after application of ketotifen fumarate to the eyelids once daily in rabbits [12], while the drug concentration at the ocular surface was stable for 24 hours after application of dexamethasone gel to the eyelids in rabbits [13]. The reason for long-term sustained activity may be slow release of the drug from skin tissue. In contrast, a high local drug concentration is not maintained by eye drops because the drops overflow when the eyes are closed immediately after instillation or the drug is excreted via the lacrimal duct.
In this study, 3 patients complained of edema, redness, and tingling of the eyelid skin as adverse reactions, while blurred vision and eye pain occurred in 1 patient each. Since the eyelid skin is extremely thin (0.55 mm), it is highly susceptible to allergic sensitization [14, 15]. Eyelid dermatitis is a common problem, and is most frequently due to allergic contact dermatitis (46 to 74%) [16, 17]. Although antibiotics have most often been reported as the causative agent, preservatives are also known to cause contact dermatitis [14]. In this study, we used Restamin Cream®, which contains a detergent (sodium lauryl sulfate) as the base. Detergents are known to cause irritation [18, 19], suggesting that sodium lauryl sulfate may have been responsible for the local adverse reactions in our patients. However, contact dermatitis due to diphenhydramine could not be ruled out since diphenhydramine allergy has been reported [20, 21].
The following limitations should be considered. This study was performed inincluded only a small number of subjects; thus, this study's findings on treatment effectiveness may not be generalizable. Clinical findings and QOL scores improved with topical treatment, but subsequent exacerbation was not observed during the washout period without medication. Allergic conjunctivitis is seasonal and the symptoms are variable, which means stable disease activity cannot be guaranteed during the study period. In addition, the washout period overlapped the end of the pollen season, making it difficult to evaluate the influence of stopping treatment. Due to incorrect recording of the VAS scores by Case 1, there were discrepancies among the clinical findings, QOL scores, and patient impressions of improvement. Although no influence of topical treatment on the intraocular pressure was detected during the short study period (2 weeks), the safety of long-term treatment is unknown. Finally, efficacy for severe symptoms is unclear because our subjects had relatively low scores for objective findings (4-10 at enrollment, with a maximum score of 60).
Further investigation of this treatment may be warranted because some patients showed marked improvement without adverse reactions, and a long-lasting effect was obtained by simple topical application of the cream. Since it is difficult for elderly persons and those with upper limb problems to use eye drops, compliance may be improved by topical application to the eyelids. No adverse reactions were reported after topical application of Vaseline to the eyelids [10], so local adverse reactions may be suppressed by modifying the base of the cream. Investigation in patients with severe symptoms, assessment of the efficacy and safety of long-term use, comparison with placebo, exploration of the optimal dose, and assessment of other antihistamines may also be considered.
In conclusion, our findings in this study suggested that application of diphenhydramine ointment to the eyelids was effective for treatment of allergic conjunctivitis. Although our results are based on a small study population, and therefore not generalizable, further investigation of topical therapy for the eyelids seems to be warranted.