INTRODUCTION
Precocious puberty is defined as the development of secondary sex characteristics before the age of 8 years in girls and 9 years in boys, which is accompanied by bone age advancement and growth spurt. Two types of precocious puberty are recognised, central precocious puberty (CPP) and peripheral precocious puberty [1]. CPP is caused by early activation of the hypothalamic-pituitary axis, with gonadotropin-releasing hormone (GnRH)- stimulated gonadotropin secretion causing gonadal maturation [2]. In peripheral precocious puberty, serum sex steroid levels are elevated independently of gonadotropin secretion, and the gonads do not undergo maturation [3]. Precocious puberty may be isosexual (involving secondary sex characteristics that are gender matched) or heterosexual (involving sex characteristics of the opposite gender). CPP is always isosexual, whereas peripheral precocious puberty may be isosexual or heterosexual [1, 2, 3].
Intracranial arachnoid cysts (ICACs) are considered benign developmental anomalies that occur within the arachnoid membrane that undergoes a splitting and traps cerebrospinal fluid inside. In most cases, they are congenital, and usually do not cause any symptoms throughout an individual's life [4, 5]. In cases in which symptoms occur, headaches, nausea, vomiting, dizziness and hydrocephalus are common. Rarely cause malformation of certain cranial bones, resulting in macrocephaly [6, 7]. A variety of additional symptoms occurs in some children depending upon the size and location of the cyst. Such symptoms include lethargy, seizures, hemiparesis, ataxia, vision abnormalities, hearing abnormalities, developmental delays, behavioral changes, cognitive impairment, and difficulties with balance and walking [4, 5, 6, 7]. ICACs can also present with variable endocrine manifestations, although only 10% to 40% of patients have CPP [8, 9, 10, 11, 12, 13, 14]. Intraventricular location is rare, account less than 1% of the total ICACs [4, 5].
The objective of this work is to carry out a literature updated review of the coexistence of CPP and intraventricular arachnoid cyst (IVAC).
EPIDEMIOLOGY
The mean annual incidence rates of CPP in girls ranging from 0.8 to 1 per 100,000 girls. The incidence in boys is approximately 10- to 15-fold lower. In Spain, the global prevalence of CPP is 0.00019 (girls: 0.00037; boys: 0.000046), with an annual incidence ranging from 0.02 and 1.07 new cases per 100,000 (girls: 0.13- 2.17; boys: 0-0.23) and an incidence rate from 1997 to 2009 of 5.66 cases per million people at risk / year (girls: 11.23; boys: 0.96). Therefore, CPP is a rare disease with a clear female predominance (approximately 10:1) [15].
ICACs account for roughly 1% of intracranial masses in newborns. The most frequent location is the middle fossa (30-60%), followed by the sellar and suprasellar region (10-20%), the quadrigeminal cistern (10%), the cerebellopontine angle (10%), and the vermis (9%). The remaining occurs in other regions. Intraventricular location is rare, in both children and adults, and represents less than 1% of the total [4, 5, 10, 13, 14, 16]. In fact, Al-Holou et al. [7] reported only 0.3% located in the ventricle among 309 cases of ICACs, and Shim et al. [6] reported only 1.4% among 209 cases.
Therefore, the coexistence of CPP and intraventricular arachnoid cyst (IVAC) is very rare in boys and exceptional in girls. The etiopathogenesis is not well recognized.
ETIOPATHOGENESIS
CPP may be idiopathic or related to a central nervous system lesion such as a neoplasm, cyst, trauma, infection, midline anomalies and hydrocephalus. CPP in girls is usually idiopathic, whereas, most cases in boys are due to an intracranial lesion [1, 2, 17]. The prevalence of intracranial lesion ranges from 30% to 70% in boys, and from 7% to 15% in girls, depending on the series [13, 16, 18, 19, 20, 21, 22, 23, 24, 25, 26].
CPP involves activation of the hypothalamic-pituitary-gonadal axis such that GnRH released from the hypothalamus causes gonadotropin secretion by the pituitary gland, leading to ovulation or spermatogenesis. The exact mechanism of a cyst's influence on the hypothalamic-pituitary axis is not completely understood. A general concept is disruption of the normal influence of the hypothalamus on the pituitary gland [27]. One hypothesis is based on mass effect of the cyst on the hypothalamus [8, 28]. Theories regarding the role of hydrocephalus in CPP include increased ventricular volume and associated mass effect on the hypothalamus, as well as direct compression of various portions of the hypothalamic-pituitary axis [27, 29].
IVACs represent simple cystic structures. The origin of these lesions is controversial, since the ventricles are lined with ependyma and not containing arachnoid membranes. Researchers have suggested that the origin seems to be secondary to the displacement of arachnoid cells by the vascular mesenchyma through the choroidal fissure during the process of choroid plexus development. In some cases, it seems to be secondary to an extension of a subarachnoid arachnoid cyst through the choroidal fissure and into the lateral ventricle [5, 30, 31].
More recently, in 2016, Knie el al. [32], analyzing follow-up neuroimages after neuroendoscopic cyst fenestration findings suggest that the IVACs were either originating from the velum interpositum cistern or from the quadrigeminal cistern. Based on this theory an arachnoid cyst that has its origin laterally may extent through the choroidal fissure to the lateral ventricle when growing. As the lateral ventricle is larger than the velum interpositum cistern, this cyst becomes more prominent. Arachnoid cysts of the quadrigeminal cistern have a close relationship to the posterior midbrain, which causes distortion or compression of the aqueduct leading to hydrocephalus, whereas in cysts deriving from the velum interpositum cistern hydrocephalus is a rare finding. Histologically, the cyst wall has arachnoid origin but can be covered with the ependymal layer when it protrudes into the lateral ventricle through the choroidal fissure. Knie el al. [32] suggest that based on the analysis of neuroimaging, it would be possible to describe the origin of an arachnoid cyst beforehand. They differentiated cysts originating from velum interpositum cistern from those originating from the quadrigeminal cistern by the long axis of the cyst. A cyst originating from the velum interpositum cistern is usually located parietal at the long axis of the ventricle. A quadrigeminal cyst extends from the midbrain in a parietal direction towards the corpus callosum. During its extension, it can grow laterally into the lateral ventricle, and thus mimicking a velum interpositum cyst. These researchers concluded that most IVACs seemed to originate from the velum interpositum cister and some seemed to extend from the quadrigeminal cistern.
DIAGNOSIS
For the diagnosis of CPP, hormone studies are needed in addition to the clinical data regarding signs of pubertal onset. Analysis of LH peaks after GnRH testing is the gold standard in the biochemical diagnosis. Imaging studies, such as bone age, pelvic ultrasound and brain magnetic resonance imaging (MRI), are also very useful. Furthermore, genetic testing must be incorporated in familial cases [21, 24]. See the diagnostic algorithm for CPP in girls (Figure 1) and boys (Figure 2).
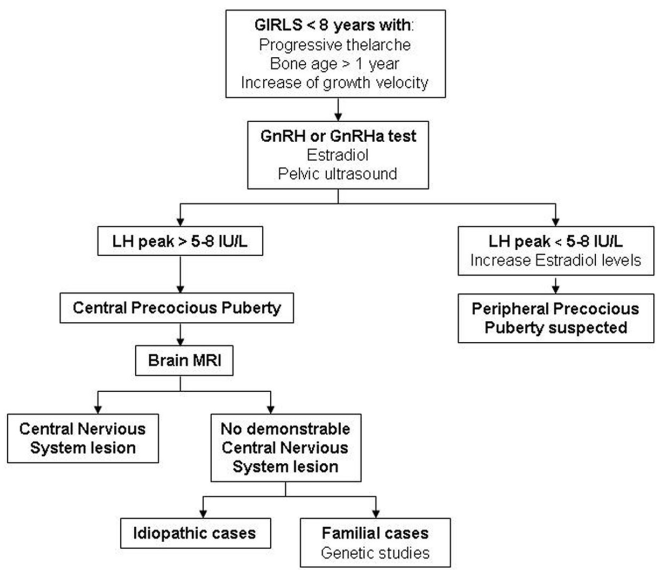
Figure 1. Diagnostic algorithm of CPP in girls.CPP: Central precocious puberty; GnRH: Gonadotropin-releasing hormone; GnRHa: Gonadotropin-releasing hormone analogue; LH: Luteinizing hormone; MRI: Magnetic resonance imaging.
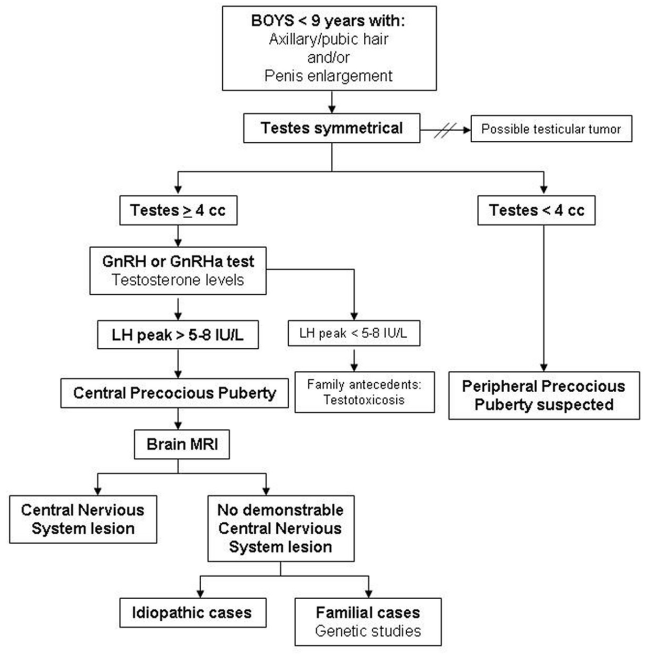
Figure 2. Diagnostic algorithm of CPP in boys.CPP: Central precocious puberty; GnRH: Gonadotropin-releasing hormone; GnRHa: Gonadotropin-releasing hormone analogue; LH: Luteinizing hormone; MRI: Magnetic resonance imaging. Importar tabla
Traditionally, CPP has been characterized by the increase of estradiol (girls), testosterone (boys) and an LH peak after stimulation with GnRH or GnRH analogues (GnRHa) (leuprolide acetate) testing in both sexes. Currently, regardless of the protocol employed, the threshold of LH peak to consider activation of puberty ranges between > 5 and 8 IU/L. A basal LH/FSH ratio of ≥ 0.2 (> 0.66 after stimulation) has been recently postulated as an indicator of pubertal activation. Nevertheless, its sensitivity and specificity do not reach that of the GnRH-stimulated LH peak. Testosterone is a useful tool in the diagnosis of precocious puberty in boys and testosterone values in the prepubertal range rule out CPP. Conversely, low estradiol levels in girls do not reject the diagnosis of CPP. In addition, although the analysis of pulsatile secretion of estradiol could be more sensitive than that of isolated estradiol determinations, this test does not achieve the utility of GnRH test in the diagnosis of CPP [1, 2, 21, 23, 24].
The bone age is notably greater than the chronological age compared to normal variants of puberty. Notwithstanding, in the early phases of CPP this advance may not be very striking. The main utility of pelvic ultrasound is to detect changes in uterine and ovarian dimensions due to estrogen exposure, and ovarian tumors or cysts that can cause an increase in estradiol production [1, 2, 24].
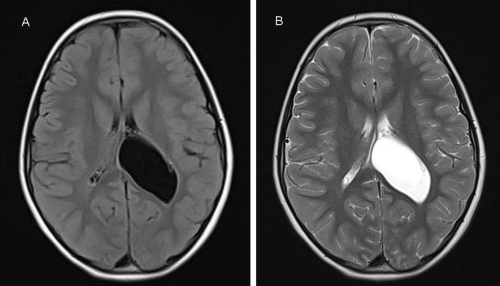
CPP is more common by far in girls than in boys. Although central nervous system disorders account for a higher percentage of cases in boys but must also be excluded in girls. Thus, girls with CPP should have a brain MRI scan as part of their assessment since clinical features, including age, are not helpful in predicting those with underlying pathology [20, 25, 26, 33, 34]. Brain MRI is the modality of choice to fully characterize ICACs. On neuroimaging, regardless of histology, arachnoid cysts are characterized as smooth, well-circumscribed lesions, with an imperceptible wall, displacing adjacent structures, and following the cerebrospinal fluid pattern (hypodense on CT and hyperintense on T2 with FLAIR suppression on MRI) (Figure 3). They can also have a remodeling effect on the adjacent bone. Arachnoid cyst in the lateral ventricle is commonly associated with focal enlargement of the lateral ventricle by the cyst with or without partial ventriculomegaly. The shape of the cyst is round or oval, not irregular [4, 5, 6, 7, 10, 13, 14, 16].
MANAGEMENT
In CPP cases secondary to central nervous system lesions, the etiological treatment, such as surgery, would not have any effect on the course of pubertal development [1, 2]. Controversy remains about when surgery is indicated and which procedures to chose. There seems to be a consensus that in pediatrics patients the indication for surgery is seizure onset, hydrocephalus, ruptured/hemorrhaged, and mass effect or slow growing clinical course [6, 32, 35].
The use of intramuscular GnRHa depot preparations (triptorelin or leuprolide) every 28 days is the medical treatment selected for CPP. This compound stems from a chemical substitution at position 6 and 10 of the native GnRH molecule, it has a decreased enzymatic degradation and in parallel, an increased affinity for the GnRH-pituitary receptor resulting in desensitization of the receptor. Consequently, this action produces an inhibition of gonadotropin secretion [36].
On the optimal age to withdraw the GnRHa treatment in girls, with the aim of menarche emerging near to the age of the normal population, we should evaluate whether to discontinue the treatment around 10 years of chronological age or 12 years of bone age. In this regard, spontaneous menses appears around 12 months after GnRHa withdrawal. In boys, we should make a decision around 11-11.5 years of chronological age. The available evidence shows that GnRHa is safe and effective, and long-term data suggests that reproductive function is satisfactory after discontinuation of treatment [24, 25, 36, 37].