INTRODUCTION
Regarding the Coronavirus disease 2019 (COVID-19) pandemic, now that widespread vaccination has helped to control outbreaks around the world, we have been left to deal with the aftermath on many fronts. As for infectious diseases, we are yet to assess what landscape of antibiotic resistance we will have to face.
Contrary to seasonal, as well as pandemic influenza, which frequently presented with concomitant bacterial lung infections, available studies regarding COVID-19 have shown an incidence of bacterial co-infections of between 7-8% in hospitalized patients, which may escalate up to 16% in those critically-ill [1]. However, estimates of antibiotic administration reached approximately 70% [2], with predominance of broad-spectrum antibiotics.
From the nationwide perspective of different countries around the globe, even in places with a marked decline in overall antibiotic consumption due to reductions in outpatient antibiotic administration, the amount of prescribed doses of antibiotics per hospital admission increased during the COVID-19 pandemic [3]. This may not only be in line with early empiric antibiotic treatment but also with the fact that COVID-19 patients have been described to have longer Intensive Care Unit (ICU) stays, with longer need for mechanical ventilation and more frequent tracheostomies [4], in close relationship to a higher rate of hospital-acquired infections [5].
Greater use of antibiotics in hospital settings, which are the niche for multidrug-resistance development, may add to the already complex situation regarding carbapenem resistance, which appears to be rising [6], and which is mainly associated with antibiotic misuse.
On top of everything, the need for new personnel and the redistribution of the existing staff to cope with the pandemic is necessary. In addition, it is required the spread of certain practices such as the use of double pair of gloves -out of fear of contagion at the start of the pandemic- and the lack of resources for Antibiotic Stewardship Programs may have pressured antibiotic resistance. However, there is an increasing albeit scarce evidence available on this matter, especially in Latin America and the Caribbean.
We therefore set out to determine whether the incidence of carbapenem-resistant isolates (CRI) from clinically relevant microbiological samples rose during the period with active cases of COVID-19 in a tertiary-care center from Santa Fe, Argentina.
MATERIAL AND METHODS
This was an analytic epidemiologic study retrospectively designed in order to assess the incidence of CRI in microbiological samples of clinical relevance, in an Argentinian hospital during the COVID-19 pandemic, compared to a previous period.
This study was conducted in Dr. JB Iturraspe Hospital, Santa Fe (Argentina), which is one of the two major hospitals in the province's capital city, which are of reference to the whole north-center region of said province. The authors acted in accordance to the Helsinki Declaration and the Hospital's Teaching Committee and the Province's Bioethical Committee approved the study.
Two periods were defined: P1 (without active cases of COVID-19) from September 2019 to August 2020 and P2 (starting at the onset of the first wave of COVID-19 in this Institution) from September 2020 to June 2021.
Inclusion criteria: All clinically relevant microbiological samples taken during the study periods from adult patients in the Internal Medicine, Surgical and ICU wards were included. We defined clinically relevant microbiological samples as all samples collected by decision of the treating physicians for diagnostic purposes, with the exclusion of rectal swabs as well as all surveillance samples.
The presence of bacterial growth from blood culture aerobic bottles was ascertained through the Bact/ALERT 3D System (bioMérieux, Argentina). A blood culture set, for the purpose of this report is considered to be composed of one to three blood culture aerobic bottles taken at once. Blood samples taken from catheter lumens were treated similarly.
Various fluids cultures (VFC) were composed of clinical samples extracted from synovial, ascitic, pleural or pericardial fluid, while various materials cultures (VMC) were composed of clinical samples extracted from abscesses and diverse tissues.
Bacterial identification and susceptibility testing were performed with the automated system Vitek 2C (bioMérieux, Argentina). Resistance to carbapenems was defined according to cutoff values on CLSI M100-ED32:2022 Performance Standards for Antimicrobial Susceptibility Testing: CIM for imipenem/meropenem ≥4 μg/ml [7, 8].
The primary aim of this study was to determine whether there was a higher incidence of CRI during P2, if compared to P1. Secondary aims were to determine: whether there was a higher incidence of CRI during P2 in ICU wards in particular; whether the positivity for Enterobacterales and NFGNB was higher in P2 and whether such isolates bore higher rates of carbapenem resistance.
Incidence density was calculated by dividing the number of CRI during each period by the count of patient-days during that same period, multiplied by a hundred. Patient-day was the sum of the in-hospital length of stay -measured in days- of individual patients during a period. 95% confidence intervals were calculated using the OpenEpi software.
Comparisons between incidences were performed using Fisher's exact test. The limit for statistical significance was a two-sided p <0.05.
Statistical analyses were performed with both OpenEpi and SPSS Statistics v27.0. Graphs were generated using Microsoft Excel 365.
RESULTS
Taken together, both periods included 9,135 hospitalizations, and 50,145 patient-days of analysis. P1 represented 62.8% of hospitalizations but only 48.7% of patient-days, with a monthly decline in hospitalizations in P2 (340 vs 478). The surgical ward, which had the leading number of admissions (60.0%), showed a decline in monthly admissions in P2 (158 vs 325). While the Internal Medicine ward showed no significant change in admissions between the periods, three new ICU wards had to be opened (totalling 6 UCI wards) during the P2 due to an increase in monthly admissions (72.6 vs 40.0). ICU mortality rose from 31.7% to 43.3%, and so did mean in-hospital stay, from 4.9 to 8.7 days. Moreover, these estimates may be biased downwards due to an increase in referral to other centers in P2 from 3.76% to 21.8%, since due to scarcity of beds in ICU wards; patients mainly remained in this center during the COVID-19 isolation period.
7,285 clinical samples were taken, the majority of which were blood culture sets (n=3,238, with a monthly estimate that raised 1.7 times in P2 at the expense of ICU wards). In second place, clinical samples from respiratory samples and catheter tips also increased by 1.9 and 2.18 times, respectively (Table 1). 1,588 Enterobacterales and 604 non-fermentative Gram-negative bacilli (NFGNB) were isolated. Monthly incidence rose in P2 overall and regarding Enterobacterales in particular (5.67 vs 3.00/100 patients-day, p<0,001 and 4.08 vs 2.20/100 patients-day, p<0,001, respectively). 62.8% of these isolates came from patients in the UCI ward.
Table 1. Number of culture samples and positivity rate for carbapenem-resistant isolates, divided by culture type and period
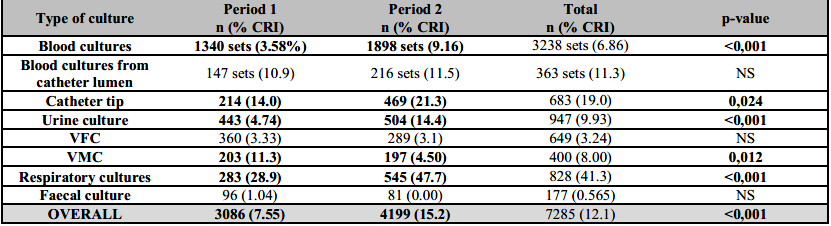
The results are presented as number (n) and percent (%).Bold letters: Significant changes in positivity rates. CRI: Carbapenem-resistant isolates; VFC: Various fluids culture; VMC: Various materials culture. NS: Non-significant.
883 CRI were isolated (80.0% from the ICU wards) from 359 patients, which barely tripled during P2 (640 vs 233). Overall CRI incidence was 1.66/100 patient-days (CI 95% 1.55-1.78). Overall CRI incidence during P2 was 2.5 times higher than in P1 (2.52 vs 0.955/100 patient-days, p <0.001). ICU CRI incidence raised from 6.78 to 8.69/100 patient-days in P2 (p=0.006) (Figure 1).
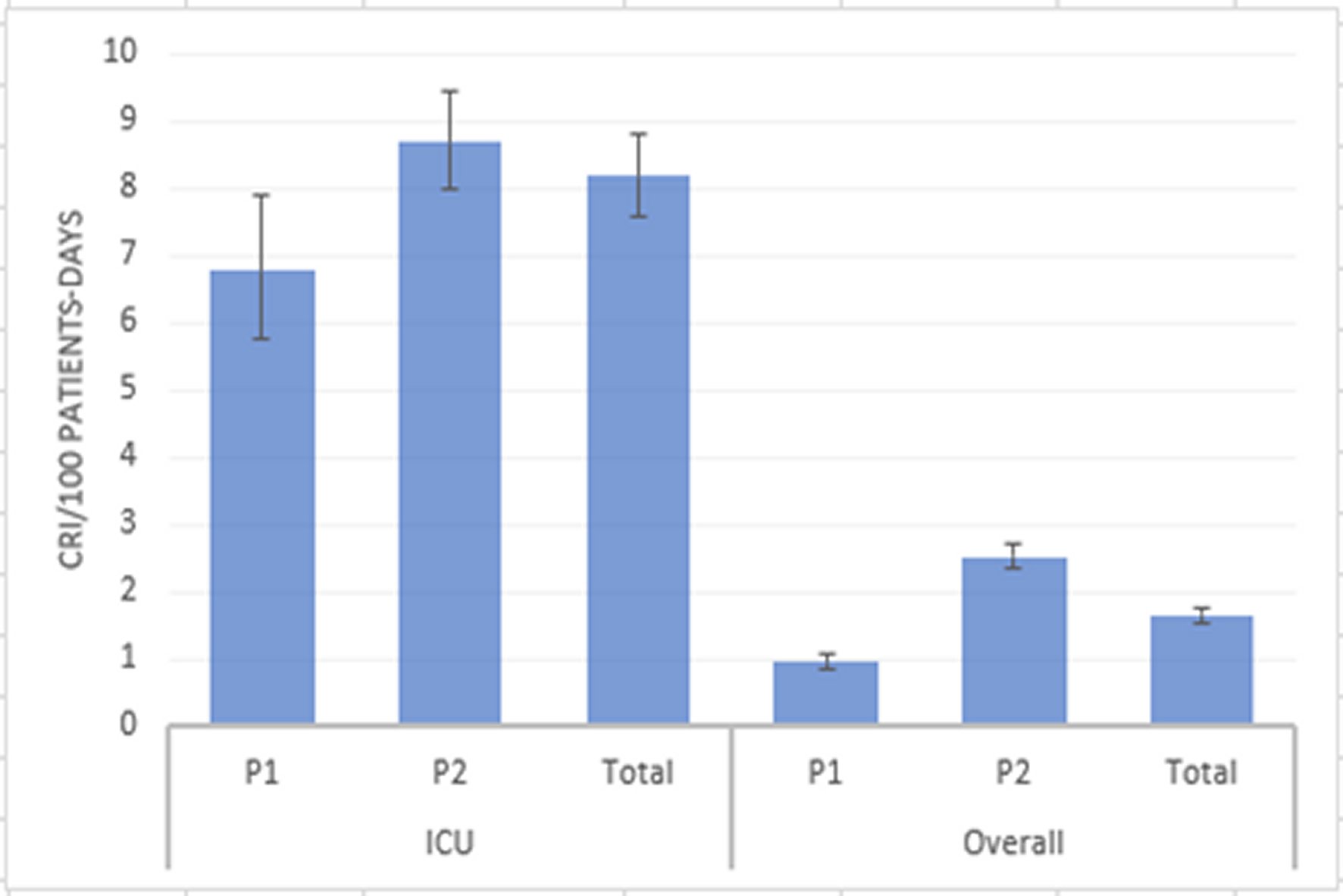
Figure 1. Comparison of incidences of carbapenem-resistant isolates between periods in the Intensive Care Unit wards and overall. Incidences are shown as CRI per 100 patients-day (blue bars) with 95% CI (black lines).CRI: Carbapenem-resistant isolates; P1: Period 1; P2: Period 2; ICU: Intensive Care Unit.
Overall blood cultures positivity to CRI rose from 3.58% to 9.16% (p<0,001), urine culture positivity from 4.7% to 14.4% (p<0,001) and respiratory samples culture from 28.9% to 47.7% (p<0,001). Other changes in positivity rates are shown in Table 1.
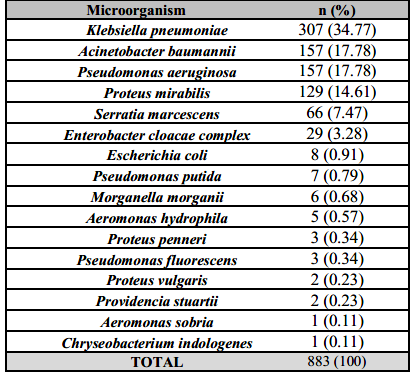
Carbapenem resistance among Enterobacterales and NFGNB rose from 30.4% in P1 to 43.9% in P1 (p<0,001). Enterobacterales represented 63.3% of CRI, with predominance of Klebsiella pneumoniae (n=307) followed by Proteus mirabilis (n=129). Among non-fermentative Gram-negative bacilli, there were 157 Acinetobacter baumannii isolates and 156 Pseudomonas aeruginosa isolates (Table 2).
Each patient with at least one CRI had a mean of 2.32 isolates during the in-hospital stay, without significant differences between periods.
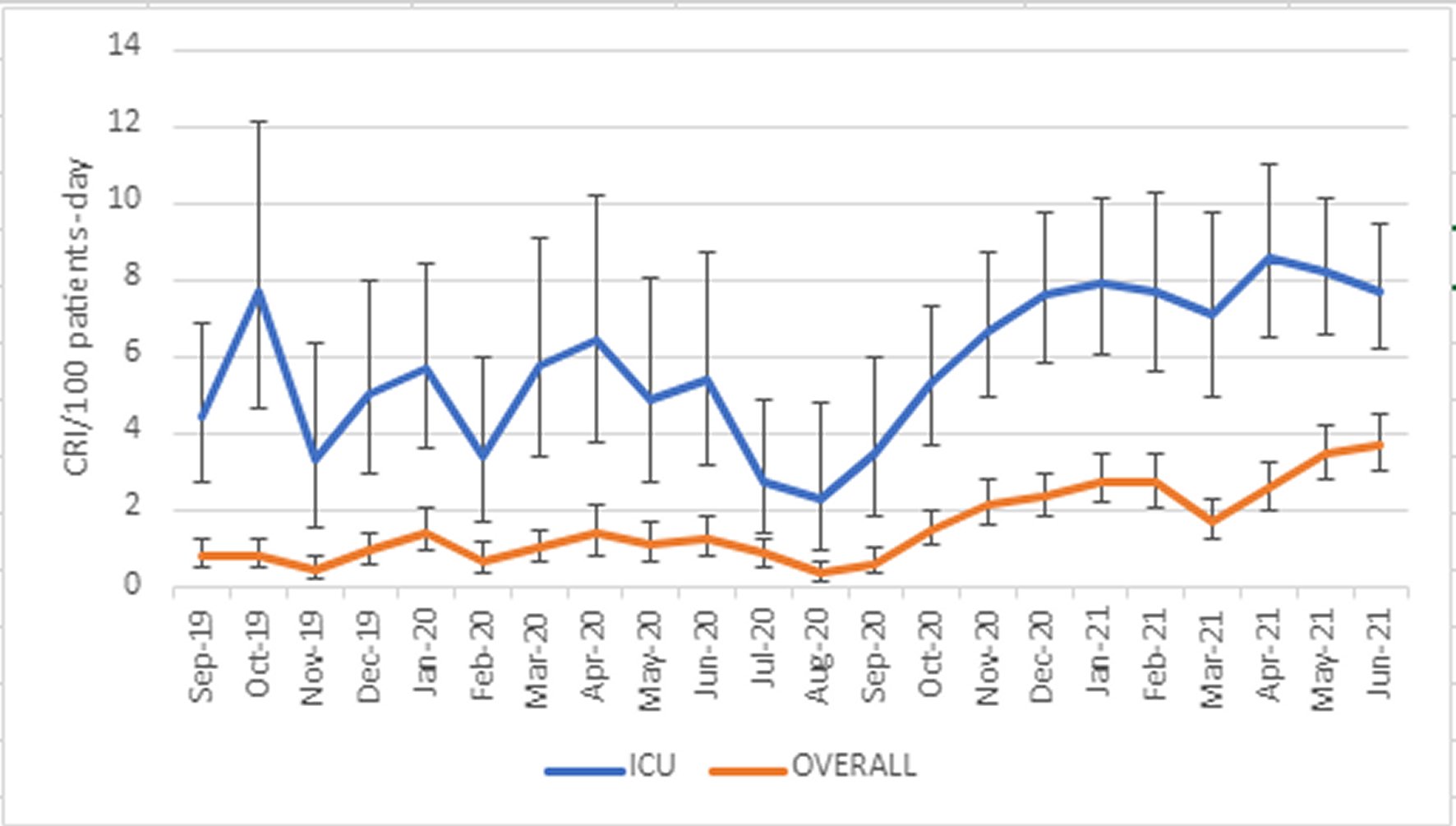
The temporal trends in monthly incidence of CRI are shown in Figure 2. Number of monthly isolates with broad confidence intervals did not permit month-to-month comparisons during P1, which showed frequent numerical changes in CRI incidence. A significant decline in the incidence of CRI developed at the end of P1 -parallel to a decrease in hospitalizations-, followed by a rapid and steady increase at the start of P1, which later stabilized.
DISCUSSION
In this study, we hypothesized that during the COVID-19 pandemic there would be an increase in carbapenem resistance incidence in our center, based on the disproportionate use of antibiotics, longer in-hospital stay and the higher ICU occupancy during this period, as well as the worldwide trend in carbapenem resistance growth.
Indeed, we found a 2.5 times increase in CRI incidence overall, at the expense of ICU patients, who not only had longer in-hospital stays but also doubled in number in P2.
Moreover, each patient with at least one carbapenem resistant microorganism isolated had a mean of 2.3 isolates, which did not significantly change between periods. Therefore, the fact that a large proportion of critical patients were referred to other centers before ICU discharge may have downsized the incidence of CRI, as opposed to what may have happened had they stayed.
Patient referral may have posed an epidemiologic threat due to dispersion of multidrug-resistant microorganisms between facilities that could aggravate the fact that in Santa Fe, Argentina, resistance to imipenem in Klebsiella sp. is already 10 points above the national estimate. However, the effect of this phenomenon has not been studied in this context. An epidemiologic study in France previous to the pandemic showed that patient transfer between facilities sustained multidrug-resistant pathogens epidemics [9].
Carbapenem resistance among Enterobacterales and NFGNB rose from a baselife of 30.4% to an alarming rate of 43.9%. This showed that the rise in CRI incidence was due not only to a higher number of patients, predominantly in ICU wards where patients tend to have longer in-hospital stays and more isolates per patient -with predominance of gram-negative bacilli-, but also due to higher carbapenem resistance among isolates. Therefore, it highlights the need for urgent Infections Control measures, reviewing what aspects during the pandemic may have influenced this rise in carbapenem resistance.
The most common CRI was Klebsiella pneumoniae, followed by Acinetobacter baumannii. Carbapenem-resistant Acinetobacter baumannii, in particular, is an especially dangerous threat to ICU patients. This was also found in one study in Wuhan, which showed that among the 6,8% of patients with secondary bacterial infections, Acinetobacter baumannii represented 35.8% and Klebsiella pneumoniae 30.8% [10].
Even in the absence of official Latin-American data on carbapenem resistance during the COVID-19 pandemic, many centers worldwide have reported a surge in CRI, albeit with heterogeneous methodologies and only a few of them with longitudinal data.
Early on in the pandemic, studies reported on episodic outbreaks of CRI, either to highlight the isolation of new strains of multidrug-resistant Enterobacterales and NFGNB or to describe the epidemiology of CRI in COVID-19 patients [11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21]. Despite the low number of bacterial co-infections at admission [22], the fact that the majority of the reports came for ICU settings coincided with the fact that bacterial infections were common during hospitalization, especially in critical patients. The high use of antibiotics in hospital settings [23, 24] along with high ICU occupancy with longer hospitalizations may justify such antibiotic resistance. Indeed, an analysis of data from the WHO Global Antimicrobial Resistance and Use Surveillance System (GLASS) reporting on 73 countries showed that around 40% of medical institutions presented a rise in hospital-acquired infections caused by multidrug-resistant pathogens during the pandemic [24]. However, limited capacity from most countries to report on antimicrobial resistance hinders conclusions from this data, which does not provide information on mechanisms of resistance or spectrum of resistance to individual antibiotics.
These reports on outbreaks of CRI led to multiple alerts from all over the world about the link of COVID-19 with antibiotic resistance [25, 26]. This was associated with high rates of antibiotic use without cultures and disruption of surveillance for multidrug-resistant bacteria [27], among other factors. Moreover, despite higher rates of compliance to hand hygiene and higher availability of personal protective equipment, there was a surge in wrong practices such as double-gloving [24].
An Italian report from 2020 showed a surge in CRI incidence, even with a strong Infections Control program and an overall decreasing trend from previous years [28]. Another multicenter before-after cross-sectional study from Italy showed a surge in carbapenem-resistant Acinetobacter baumannii incidence, albeit without changes in the incidence of carbapenemase-producing Enterobacterales [29]. Similar to this, a New York City center showed a tendency to a greater incidence of CRI in COVID-19 patients, after a steady decline in previous years [11]. The majority of isolates in this report were from respiratory samples, similar to what happened in our center.
A Spanish study showed that in the same period COVID-19 patients had two times the incidence of CRI if compared to control patients (1.1 vs 0.5%) [15].
Regarding Latin-American countries, one prospective cohort study from a tertiary care center in Mexico City that included 794 patients with severe COVID-19, identified 110 hospital-acquired infections in 74 patients, the majority of which (69.6%) were caused by Enterobacterales, however with a low prevalence of carbapenem resistance [30].
A multicenter study regarding 46 Mexican centers (40 hospital-based laboratories and 6 external laboratories from the network Red Temática de Investigación y Vigilancia de la Farmacorresistencia-INVIFAR) monitored antibiotic resistance among critical and high-priority microorganisms. They found a surge in carbapenem resistance in Klebsiella pneumoniae and Escherichia coli isolates in blood, urine and respiratory samples, Pseudomonas aeruginosa isolates among respiratory and urine samples and Acinetobacter baumannii isolates among respiratory samples [31].
Meanwhile, a Brazilian study recently found a surge in carbapenem as well as polymyxin B resistance among healthcare-associated infections in the ICU of a tertiary care hospital during the post-pandemic period [32].
In October 2021, the Panamerican Organization of Health published a document warning about the emergence and increment of carbapenemase-producing Enterobacterales in Latin America. There, it highlighted the first report of Enterobacterales co-producing KPC and NDM carbapenemases in Argentina -as well as in other countries- and a three times increment in KPC and NDM producing Enterobacterales in Uruguay [33].
A Brazilian study using aggregate data from 99 hospitals from Paraná that reported 11,248 device-associated infections in 234,631 patients admitted to ICU between January 2019 and December 2020 reported a significant increase in the proportion of carbapenem-resistant Acinetobacter baumannii isolated in 2020 (12.4% vs 7.9%). In the trend analysis, the monthly incidence density of carbapenem-resistant Acinetobacter baumannii per 1000 patient-days increased significantly, while carbapenem-resistant Klebsiella pneumoniae per 1000 patient-days showed a gradual increase during the entire observed period, but with no change in trend [34].
Several factors may have favoured the rise of CRI incidence in our, as well as other, centers. Limited resources worldwide, together with high patient burden, may have resulted in difficult decision-making, limiting the use of gloves and gowns to certain situations. Moreover, the use of personal protective equipment created a false sense of security for the personnel tending to cohorted COVID-19 patients. This false sense of security is a particularly dangerous epidemiological threat, since it has already been shown that there is a high likelihood of carriage of carbapenem-resistant Enterobacterales in gowns and gloves that come in contact with carriers of CRI [35].
Even more, during the COVID-19 pandemic, contact and respiratory isolation for all patients posed a significant burden on the health personnel. A high percentage of patients in contact isolation has shown to reduce compliance to hand hygiene, among other contact precaution measures [36]. Overcrowding, under-staffing and a high patient-caregiver ratio -all common during outbreaks of COVID-19 pneumonia- have been associated with a higher risk of cross-transmission [37].
CONCLUSIONS
In the midst of the COVID-19 pandemic, several factors were thought to favour outbreaks of carbapenem resistance, which had already been declared a worldwide emergency. Indeed, we found alarming rates of CRI in our center, 2.5 times higher than before the first COVID-19 wave, similar to other reports worldwide.
To our knowledge, to this date this is one of the few Latin-American studies on the effect of the COVID-19 pandemic on CRI incidence, reporting on over 800 CRI among over 50,000 patient-days of analysis. Indeed, we found alarming rates of CRI in our center, 2.5 times higher than before the first COVID-19 wave, similar to other reports worldwide.
More studies are needed to understand the real trend in carbapenem resistance and whether unified efforts in infectious control measures will be able to manage these outbreaks in the aftermath of this pandemic.