INTRODUCTION
Bleeding is an important complication that increases morbidity in patients after transurethral prostate resection (TUR-P) [1]. Prostate volume, amount of resected prostate tissue, operation duration, urine culture positivity, anaesthesia type, medications used before the operation, patient age and blood pressure have been associated with perioperative blood loss [2]. Apart from these, there are studies showing that the activated fibrinolytic system in the blood has an effect on bleeding [3].
The fibrinolytic activator has been found in many tissues of the human body [4]. Prostate tissue itself has been reported to be one of the tissues with the highest concentration of this activator [4], and fibrinolytic activators have been found to be contained in the urine [4]. Both the level of urokinase in the urine and plasminogen activators in prostate tissue play a role in the fibrinolytic process and hence lead to blood loss in the perioperative period [4].
Many studies on systemic and local agents for fibrinolytic activity with conflicting results have been reported [5, 6, 7, 8]. Considering the side effects of the agents, in this study, we aimed to investigate the effects of the local dilution of fibrinolytic agents with manual irrigation on postoperative bleeding and surgical outcomes.
MATERIAL AND METHODS
Between April 2017 and April 2021, a prospective, single-blinded randomised trial was conducted with men requiring TUR-P for obstructive urinary symptoms. Local ethics committee approval before starting the study (Registration number: 2017-03-11/01) and written informed consent from all participants were obtained.
Inclusion criteria were presentation with lower urinary tract symptoms nonresponsive to medical treatment or refractory or chronic urinary retention due to benign prostatic hyperplasia (BPH) and prostate volume < 100 g.
Exclusion criteria included evidence of prostate disease other than BPH, any previous open or endoscopic prostate surgery, treatment with any 5ARI within 12 months, requirement for treatment with any anticoagulants during the restricted periods, prostate volume > 100 g, urinary infection and severe comorbid conditions (such as liver, cardiovascular and haematological diseases).
After power analysis, 128 patients meeting the criteria were included in the study. Similar pre-treatment assessments and procedures were performed for all participants to establish the diagnosis of bladder outlet obstruction due to BPH, including the assessment of voiding efficacy quantified by maximum flow rate (Qmax), postvoid residual urine volume (PVR) and International Prostate Symptom Score (IPSS). Prostate size was measured using transrectal ultrasonography (TRUSG) by a senior staff member. When indicated, a TRUSG biopsy was first taken to exclude malignancy.
Patients were randomised into two study groups in a 1:1 ratio by coin toss. In the first group (control group), a urethral catheter was inserted after the operation, and continuous irrigation with isotonic saline solution was started on the operation table. In the second group (intervention group), after the insertion of the urethral catheter after the operation, the bladder was manually washed with 1000 cc of saline at room temperature with a pine-tip injector through the urethral catheter before continuous irrigation with isotonic saline solution was started. This was a single-blinded study due to the nature of the study. Patients were blinded to the intervention type but blinding of the treatment provider and outcome assessor could not be done.
Patients taking anticoagulant medication discontinued their treatment at appropriate time intervals before surgery. All TUR-Ps were performed under spinal anaesthesia in a lithotomy position and by a senior staff member. During the operations, a standard monopolar 26 F resectoscope and irrigation fluid at room temperature were used. The operative duration and amount of saline used for irrigation during the operation were calculated for each patient. The operation time was defined as the period from the beginning of the resection until the end of haemostasis. After weighing, all resected tissue underwent pathological evaluation.
The day after the operation, the irrigation fluid was stopped when it became clear. The amount of fluid used for each patient was calculated, and to determine the total haemoglobin (Hb) loss, another blood sample was taken. Since bleeding was considered insignificant after 48 h [3], it was not necessary to measure late Hb values in patients in whom we did not observe gross bleeding. Surgical blood loss during and after the operation was calculated by the red blood cell (RBC) method as described in the study by Ukimura et al. [1]. The patients were discharged when they could urinate successfully after the removal of their urethral catheters. For each patient, catheter duration and hospital stay were recorded. The first-month follow-up visits for patients were done using the IPSS, QoL, Qmax and PVR.
The sample size was determined by the G-Power 3.0.10 Program aimed at 95% power and 0.05 alpha error values, considering the blood loss level presented in a reference article by Lira-Dale et al. [9]. Results showed that the minimum sample size was 52 for each group and 104 in total (critical t=1.6596374; p<0.05). Nominal and ordinal parameters were described with frequencies, whereas scale parameters were described with means and standard deviations (SD). Fischer’s exact and chi-square likelihood ratio tests were used for differences between categorical variables. The Kolmogorov–Smirnov test was used for normality analysis of scale parameters. The Mann–Whitney U test was used for differences between non-normally distributed parameters, and the independent samples t-test was used for differences between normally distributed parameters. SPSS 17.0 for Windows was used for analysis at 95% confidence interval (CI) with a 0.05 significance level.
RESULTS
The mean age of the patients was 66.88±8.26 years, mean IPSS was 22.63±6.62, mean Qmax was 6.67±3.76, mean PVR was 301.29±277.78 mL, mean prostate volume was 51.65±17.47 cm3, mean Hb was 13.62±1.46 and mean PSA was 3.53±3.10, respectively. There was a non-significant difference in all pre-operative data between the groups (p > 0.05) (Table 1).
Table 1. The baseline characteristics between two groups
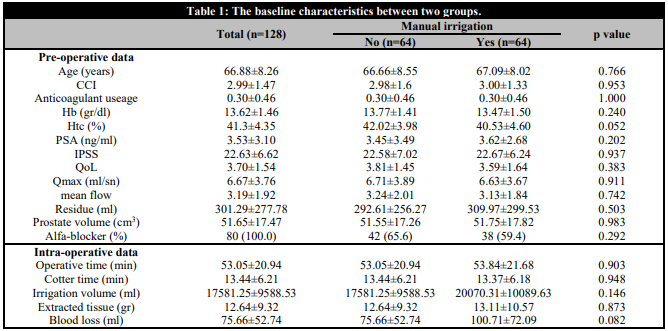
CCI: Charlson comorbidity index; Hb: Haemoglobin: Htc: Haematocrit; PSA: Prostate-specific antigen; IPSS: International prostate syndrome score; QoL: Quality of life.
The mean resected prostate weight was 12.64±9.32 g in the control group and 13.11±10.57 g in the intervention group. Operation time was 53.05±20.94 minutes and 53.84±21.68 minutes, respectively. Cautery time was 13.44±6.21 minutes and 13.37±6.18 minutes, respectively. The irrigation fluid used was 17581.25±9588.53 mL and 20070.31±10089.63 ml, respectively. There was no statistical difference between the two groups (p > 0.05) (Table 1).
The mean Hb loss within the first 24 h after TUR-P is shown in Table 2, and there was no significant difference in total Hb loss (perioperative) between the groups either. Gross bleeding was not observed in any patient after the operation, the need for blood transfusion was not observed and retention was not observed in any patient after catheter removal. There was no significant difference between the groups in the volume of saline used for bladder irrigation, the catheter dwell time and the length of hospital stay (Table 2).
Qmax, PVR, IPSS and QoL values in the first month for the patients were statistically similar between the groups (Table 2).
Table 2. Comparisons of post-operative data between two groups.
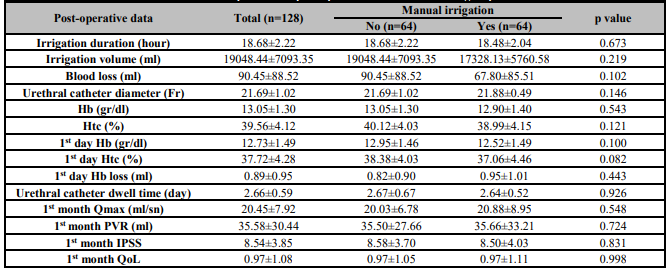
Hb: Haemoglobin: Htc: Haematocrit; IPSS: International prostate syndrome score; QoL: Quality of life; PVR: Postovoid residual urine volume.
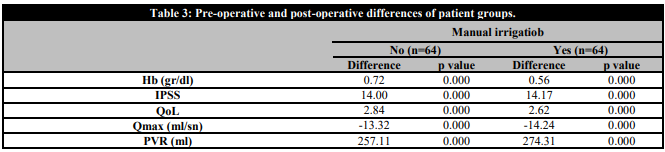
The differences in preoperative and postoperative data according to the groups are shown in Table 3. It is seen that the differences between the groups are in favour of the intervention group. No malignant findings were found in the pathologies of any patient.
DISCUSSION
TUR-P remains the gold standard for correcting bladder outlet obstructions in medium-sized prostate volumes [2]. Due to the complications it contains, it is a procedure that requires hospital follow-up. Perioperative bleeding is the most troubling complication [10]. In one study, severe bleeding was reported to be at a 13% rate [11]. This procedure carries the risk of haemodynamic instability due to severe bleeding, reoperation and prolonged hospitalisation.
Catheter traction has been attempted to reduce perioperative bleeding. Agents such as oestrogen, vasopressin, fibrin sealants and phenol solution have been applied both parenterally and locally, but no agent or technique has been accepted in clinical routines [12, 13, 14, 15, 16, 17].
Lira-Dale et al. injected transurethral epinephrine into the prostatic tissue prior to TUR-P and observed a reduction in the amount of bleeding [9]. Although it is applied locally, it carries the risk of invasiveness from the application process and the need for haemodynamic follow-up in the patient. In another study, Khorrami et al. reported that by injecting fibrin glue, a combination of fibrinogen and thrombin into the prostatic bed after resection decreased bleeding, and this would not have a negative effect on lower urinary tract system symptoms [10]. Although the application is superficial, the need for fluoroscopic control creates an additional radiation risk to the patient. Considering these difficulties in the application of agents used for haemostasis, our application seems to be an easier procedure. However, we did not see a statistical difference between the groups in terms of postoperative blood loss, Hb reduction and duration of hospital stay.
Studies evaluating the relationship between systemic fibrinolytic activity and post-TUR-P bleeding in the literature could not reveal this relationship sufficiently [18, 19, 20, 21]. This situation, which is attributed to methodological inadequacies, was attempted to be overcome with the study designed by Nilsen et al. [3], but they also could not establish a relationship between systemic fibrinolytic activity and post-TUR-P bleeding. Then, Nilsen et al. attempted to elucidate the relationship between local fibrinolysis and post-TUR-P bleeding, and the existence of such a relationship was emphasised [22].
A robust haemostatic system is essential for wound healing [23]. Bleeding control may not be easy due to vessels being opened in the resection area in prostate surgery. The blood clot formed in a local area is a response to prevent bleeding [23]. Tissue plasminogen activators in the prostate capsule and urokinase in the urine can increase existing bleeding by causing the destruction of formed clots and may interfere with the healing process [23]. Therefore, we attempted to prevent this cycle by washing the prostate gland with saline to dilute the fibrinolytic agents released. However, we could not show the superiority of manual irrigation in haemostasis in our study.
Our application did not show the change in concentrations of local fibrinolytic agents, as we did not directly measure fibrinolytic activity in the local setting. This was a limitation of our study. In fact, our aim was to demonstrate the clinical reduction of bleeding. Another limitation was that although we could not show a difference between the groups clinically and biochemically, we could not explain the serum changes due to the absorption of the saline we applied locally. In addition, when we compared the first-month data of the patients with the preoperative data, we saw that the improvement in the intervention group was greater. It was not possible to explain this situation with the available data. At the third and twelfth month evaluations, which will show whether this situation continues in the long term, will enable us to make a better interpretation.