1. INTRODUCTION
Within the natural course of infection caused by hepatitis B virus (HBV), there is a complex interaction between the virus and the immune response of the host [1]. T-cells significantly decrease from the early stage of pregnancy until the 20th week, reducing the level of immunity. It has been demonstrated that during pregnancy, increased hormone levels of progesterone, oestrogen, and human chorionic gonadotropin significantly suppress cell-mediated immunity. CD8 T-cells specific to HBV play a very important role in clearing acute HBV infection, but during pregnancy, the responses of T-cells specific to HBV have been shown to be weaker or unresponsive compared to non-pregnant patients [2]. In the period close to the birth, the cortisol level reaches a peak, and the sudden drop after the birth can cause HBV reactivation by showing similar effects to the termination of steroids. During the peripartum period, responses may occur in T-cells specific to HBV [3].
A study conducted in Turkey covering the period 2010-2018 reported hepatitis B surface antigen (HbsAg) prevalence in pregnant women to be 1.6%-1.8% [4], and in a later 2018 meta-analysis, this rate was determined to be 2.84% for cumulative HbsAg [5]. Although there are limited data showing how pregnancy affects chronic HBV infection, it has been reported that there is no worsening of liver disease associated with HBV during pregnancy [6]. There are also studies in literature that have stated that chronic HBV can be reactivated during pregnancy and severe HBV flare up result in liver failure [7, 8]. The aim of this study is to investigate HBV reactivations through examinations of HBV DNA and serum alanine aminotransferase (ALT) levels throughout pregnancy and in the postpartum period of women diagnosed with chronic HBV (CHB) infection who had not received treatment before pregnancy.
2. MATERIAL AND METHODS
2.1. STUDY PROTOCOL
The sample of this retrospective study included adult (>18 years) pregnant CHB patients not receiving treatment in the Infectious Diseases and Clinical Microbiology polyclinics of 5 centres in the east and southeast of Turkey between 2017 and 2022. Medical records of pregnant women with a diagnosis code of hepatitis B were obtained from the hospital database and examined. To be able to evaluate hepatic reactivation, patients with results of at least two periods of prepartum, intrapartum and postpartum periods were included. The aspartate aminotransferase (AST), ALT, bilirubin, HBsAg, hepatitis B surface antibody (anti-HBs), hepatitis B e antigen (HBeAg), hepatitis B e antibody (anti-HBe), and HBV DNA levels of the pregnant women in the prepartum, intrapartum (3rd trimester), and postpartum periods were recorded separately on a patient data form.
The exclusion criteria of the study were defined as the presence of acute HBV, other viral infections (hepatitis C virus, hepatitis D virus, human immune deficiency virus), a history of chronic liver disease, complications related to the pregnancy (intrahepatic cholestasis of pregnancy, HELLP syndrome, pre-eclampsia, placental bleeding), drug use other than dietary supplements of iron, folic acid, calcium, and other vitamins, the presence of bacterial, fungal, or parasitic infections, or previous treatment for CHB.
2.2. DEFINITIONS
Viral reactivation; An increase of ≥2 log in the HBV DNA levels compared to initial values or HBV DNA at the level of >100 IU/ml in an individual with no HBV DNA determined initially.
Biochemical reactivation: An increase of 5-fold more than the upper limit of ALT or a 3-fold increase from the basal value [9].
2.3. CHB PHASES
Phase 1: HBeAg-positive chronic HBV infection: defined as serum HBeAg, HBV DNA at a very high level, and ALT at a chronic normal level (≤40 IU/mL).
Phase 2: HBeAg-positive chronic hepatitis B: defined as serum HBeAg, HBV DNA at a high level, and elevated ALT.
Phase 3: HBeAg-negative chronic HBV infection: defined as serum antibodies against HBeAg (anti-HBe), HBV DNA which is not detectable or at a low level (<2.000 IU/mL), and ALT at a normal level (≤40 IU/ mL).
Phase 4: HBeAg-negative chronic hepatitis B: defined as anti-HBe, which can usually be detected, and the absence of serum HBeAg, persistent or fluctuating moderate-to-high serum HBV DNA levels (generally lower than in HBeAg-positive patients), and fluctuating or per-sistently high ALT levels.
Phase 5: HBsAg-negative phase: defined as serum HBsAg negativity and the presence of anti-HBc positivity, either with or without de-tectable antibodies against HBsAg (anti-HBs). The presence of a low level of HBV DNA positivity is known as “occult HBV infection” [10].
2.4. STATISTICAL ANALYSIS
Statistical analyses of the study data were applied by using SPSS for Windows vn. 20 software (SPSS Inc., Chicago, IL, USA). Descriptive statistics were presented as mean ± standard deviation (SD) or median, minimum, maximum values for continuous data and as number (n) and percentage (%) for categorical data. Comparisons between groups of categorical data were analyzed by using the Chi-square test and if there were <5 units in cells, the Fisher Exact test. As the continuous data did not meet parametric assumptions, the non-parametric Kruskal Wallis and Mann Whitney U-tests were applied in comparisons. To determine the cutoff value of HBV DNA for the development of reactivation, Receiver Operating Characteristic (ROC) curve analysis was performed. The best cutoff value was determined using the Youden Index, and to determine the diagnostic value of risk factors, the area under the curve (AUC) was calculated from the ROC analysis. A value of p<0.05 was accepted as the level of statistical significance.
3. RESULTS
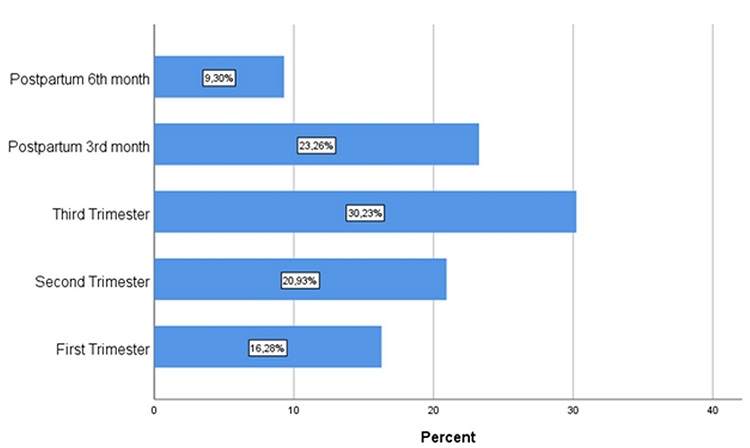
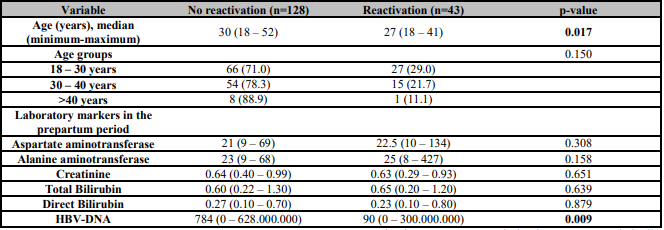
171 pregnant patients with CHB with a median age of 30 years (range, 18-52 years) were evaluated. Hepatic reactivation developed in 43 (25.1%) patients, in the postpartum period in 14 (32.35%) of these patients and in the intrapartum period in 29 (67.44%). Reactivation occurred most often in the 3rd trimester (n:13, 30.2%). The frequencies of the development of reactivation during the follow-up period are shown in Figure 1. Comparisons were made of the epidemiological and laboratory test results of the patients who developed and did not develop reactivation (Table 1). The patients who developed reactivation were seen to be younger than those who did not (p=0.017). The HBV DNA level in the prepartum period was observed to be significantly lower in patients who occurred reactivation.
Table 1: Comparisons of the basic characteristics of the patients according to the reactivation development status.
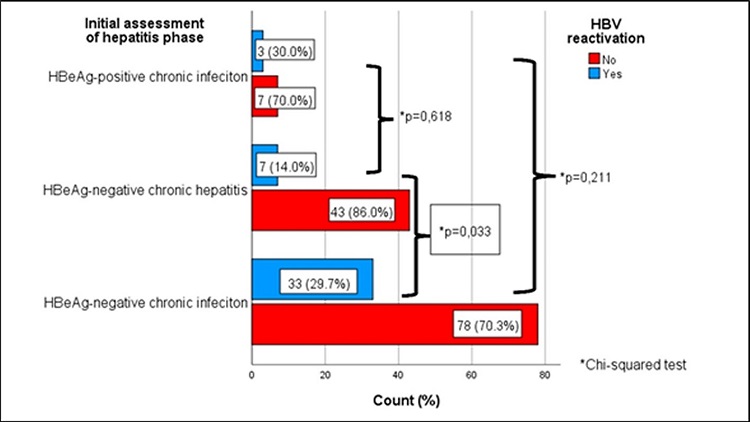
The CHB phases in the prepartum period of the pregnant patients in the study are shown in Figure 2. According to this, 111 (64.9%) patients were determined to be in the stage of HBeAg negative chronic infection, 50 (29.2%) in HBeAg negative chronic hepatitis, and 10 85.8%) in HBeAg positive chronic infection. Reactivation was determined more often in the patients with initial HBeAg negative chronic infection than in those with HBeAg negative chronic hepatitis (p=0.033). There was no statistically significant difference between the CHB phases in the prepartum period (Figure 2).
The changes over time in the ALT and AST values according to the status of HBV reactivation development, and the treatment status of those who developed reactivation are presented in Figure 3. An increase of a statistically significant level was determined in the ALT levels of the patients with viral reactivation between the 6th month prepartum and the second trimester (p=0.037, p=0.017). In the postpartum period, the ALT values of those who developed viral reactivation decreased significantly (p=0.010). The HBV DNA levels according to the treatment status of patients with viral reactivation are shown in Figure 4. In the patients with increasing level of HBV DNA in the 3rd trimester and who were not treated, the level was seen to regress in the postpartum period. There was a statistically significant increase in the AST levels of the patients with and without viral reactivation between the 6th month prepartum and the first trimester (p=0.038, p=0.039).
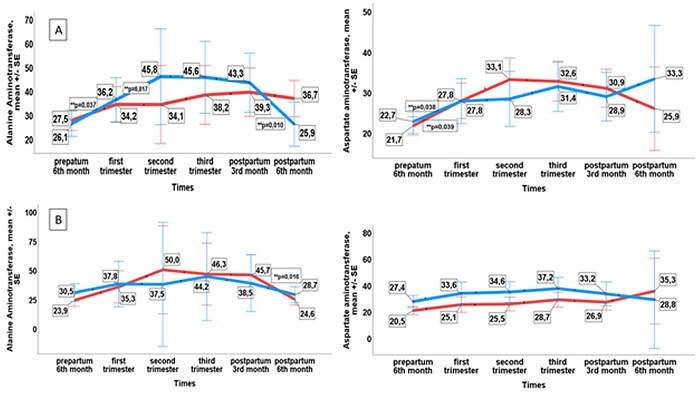
Figure 3: (A) the change in ALT and AST values from prepartum to 6 months postpartum according to the reactivation development status, and (B) the change in ALT and AST values from the prepartum 6th month to 6 months postpartum according to the treatment status of those who developed reactivation.
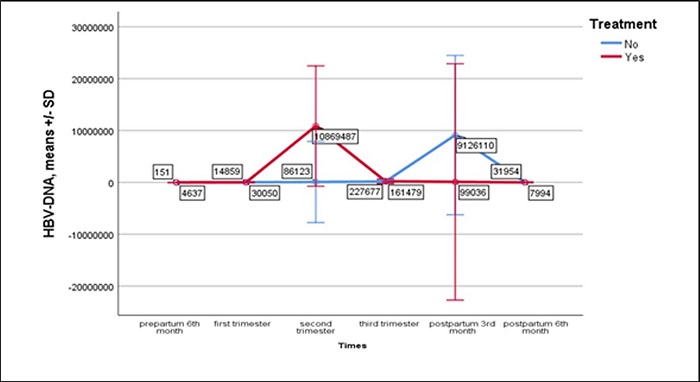
Figure 4: The course of HBV DNA according to the treatment status in patients who developed reactivation.
Of the 43 patients who developed reactivation, 10 (23.3%) received treatment, and 33 (76.7%) did not receive treatment. The ALT value decreased statistically significantly less in the postpartum period in the patients who did not receive treatment compared to those who were treated (p=0.016). During follow up, no significant change was observed in respect of the AST value in patients who developed reactivation, independently of treatment status.
Of the 10 patients who occurred reactivation and started treatment, the treatment was started in the 3rd trimester in 5 patients, in the 2nd trimester in 4, and in the 1st trimester in 1. Of the 5 patients who started treatment in the 3rd trimester, the HBV DNA level was seen to be >200,000 in only 2 patients. No clinical symptoms were determined in any patient who developed reactivation.
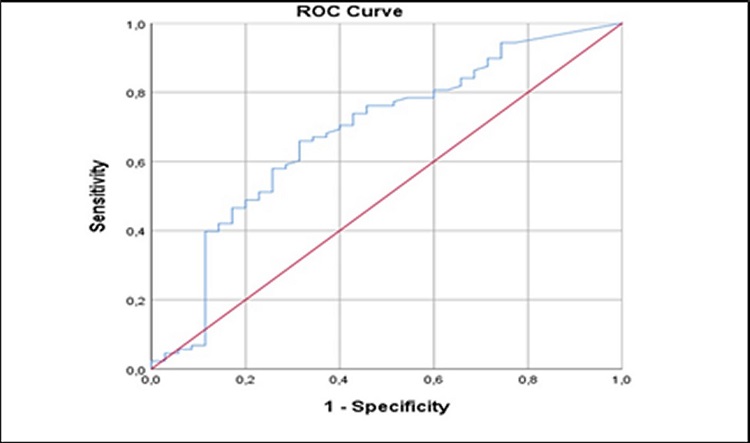
The ROC curve of the prepartum HBV DNA (IU/ml) of the patients with initial HBeAg negativity was shown in Figure 5. The prepartum HBV DNA level (cut-off point =192 IU/ml) of patients with HBeAg negativity was found to have diagnostic power for reactivation of 0.684 (95% CI:0.575-0.792, p=0.002) with 65.9% sensitivity and 68.6% specificity.
In one patient who developed hepatitis B reactivation in the first trimester, anti-HBs seroconversion developed in the postpartum period.
4. DISCUSSION
Hepatitis B in pregnancy is an important problem for both the mother and the fetus. The liver problem most often seen in pregnancy is hepatic reactivation. Attention must be paid to pregnant patients in respect of reactivation in both the intrapartum and postpartum periods. In a pregnant patient with stable disease, not at an advanced stage with antiviral treatment postponed, the newborn is at risk of perinatal hepatitis B towards the third trimester. There should be an evaluation at the end of the second trimester of the benefits and risks of starting treatment by measuring the HBV DNA level [9]. For all these reasons, HbsAg positive pregnant patients should be followed up at regular intervals.
Acute exacerbations of varying degrees can be seen in healthy HBV carriers and those with CHB infection for several reasons, including pregnancy. Most of the studies in literature are related to vertical transmission in pregnant women diagnosed with CHB, and it is striking that there are few studies related to hepatitis B reactivation in pregnancy. In a study that included 29 pregnant women diagnosed with CHB, there was reported to be an increase in the HBV DNA level both in pregnancy and in the early postpartum period [8]. In a prospective study of HBeAg negative pregnant women, reactivation was seen to develop in 30% of the patients in the first 6-month period postpartum. Of the 41 patients with reactivation, the prepartum HBV DNA level was determined to be >2000 IU/ml in 14 (34.1%), <2000 IU/ml in 18 (43.9%), and the level could not be determined in 9 (21.9%). HBV reactivation did not develop in any of the pregnant women with HBV DNA not determined in the prepartum period. In the same study it was shown that the prepartum HBV DNA levels were >10,000 IU/ml in the majority (62.5%) of the pregnant patients who developed hepatic reactivation. The pregnant women in that study were evaluated in the 28th-32nd week of pregnancy and then postpartum reactivation was examined [11]. The current study was not restricted to only one period, and hepatic reactivation was also evaluated in the intrapartum and postpartum periods. Reactivation was seen to develop in the postpartum period of 14 of 43 patients in the current study, and in the intrapartum period in 29. Previous studies have reported that although hepatic reactivation developing in pregnant patients is usually limited to the existing clinical status and does not require treatment, it can sometimes lead to severe clinical conditions such as liver failure [12].
No clinical symptoms were determined in any of the current study patients, which was consistent with data in literature. It was thought that starting treatment in the intrapartum period could have overcome reactivations, which could potentially show clinical symptoms in the postpartum period. Of the current study patients who developed intrapartum reactivation, 10 were treated, starting in the 3rd trimester in 5 patients, in the 2nd trimester in 4, and in the 1st trimester in 1. Of the 5 patients who started treatment in the 3rd trimester, the HBV DNA value was seen to be >200,000 in only 2. Although treatment is recommended for pregnant patients with HBV DNA level>200,000 IU/ml in the 3rd trimester [13, 14], treatment was also started for patients with a lower HBV DNA level in the current study. In one patient infected with HBV in the first trimester, the evaluation of previous underlying liver disease was necessary. If the patient is disease immune tolerant or if it is in the early stage, treatment can be postponed to the 3rd trimester to reduce the risk of vertical transmission. It has been reported in previous studies that starting treatment can be considered in advanced stage disease (high ALT and HBV DNA levels and/or the presence of hepatic fibrosis) which has not been previously detected [15, 16, 17]. In addition, another approach that is supported is to start antiviral treatment for pregnant patients who have previously given birth to an infant with hepatitis B, even if the HBV DNA levels are low [18]. The reason for starting treatment in all the current study patients was not known, as the study was retrospective.
In a previous study, HBV DNA was determined to increase in pregnancy and decrease after the birth. In the same study, it was reported that reactivation during pregnancy may not be able to be predicted using HBV DNA, ALT level, HBeAg status, or any other risk factor [19]. In the current study patients who developed viral reactivation in the intrapartum period and did not receive treatment, the HBV DNA level spontaneously regressed in the postpartum period. In another study, it was determined that the HBV DNA levels of HBeAg negative pregnant patients were mean 6 log lower than in HBeAg positive patients, and despite a relatively stable course in pregnancy, a 1 log increase was seen in 25% of patients [20]. In the current study, HBV DNA was seen to be higher in HBeAg positive patients and reactivation rates were higher in the HBeAg negative patients.
Wang et al. reported that ALT exacerbation was seen when the HBV DNA examined at birth was determined with a cut-off value of 5 log 10 IU/ml [21]. In another study, ALT and HBV DNA examined during pregnancy were seen to be correlated in univariate analysis for postpartum ALT reactivation, but this was not supported in multivariate analysis [22]. In the current study, the cut-off value for HBV DNA examined in the prepartum period was determined as 192 IU/ml with 65.9% sensitivity and 68.6% specificity for hepatic reactivation.
In a study of women in Singapore, an increase in ALT was reported in the peripartum period in those with chronic HBV infection and there was seen to be more HBeAg seroconversion than in non-pregnant women [23]. In one patient with CHB infection in the current study, reactivation developed in the first trimester, and HbsAg seroconversion developed later. The HBV DNA value in this patient was 10 IU/ml before pregnancy, 11,500 IU/ml in the first trimester, then became negative in subsequent trimesters, and in the examination at 6 months postpartum, HbsAg was negative and anti-HBs was positive.
ALT exacerbations in pregnant women infected with HBV have been reported to occur in 9-45% of women, mostly within 3 months of delivery [24, 25]. In a study that examined 146 pregnant patients infected with chronic HBV, who had not received antiviral treatment, a significant increase was seen in the median ALT levels from baseline 17 U/L to 29 U/L postpartum [26]. In a study of US women not receiving antiviral therapy before pregnancy, only 6% of patients with ALT exacerbations during pregnancy had a ≥2 log increase in HBV DNA [27]. In the study of Kim et al., it was found that 50% of women who received antiviral treatment before pregnancy and stopped the treatment when pregnancy was diagnosed had ALT reactivation, 67% had HBV reactivation, and then spontaneous recovery was observed in all of them [28]. The ALT level was also observed to have significantly increased in the current study patients who developed HBV reactivation. Although there was an increase in ALT in our study, none of them were considered as reactivation. The AST value was seen to increase in the postpartum period independently of reactivation.
5. LIMITATIONS
Some limitations of this study should be considered. First, the data of some patients were incomplete as the study was retrospective in design. Another limitation was that other than for patients in the 3rd trimester, there were no data related to the reason for starting treatment.
CONCLUSIONS
In conclusion, HBV reactivation may occur during pregnancy or postpartum in women with CHB. In our retrospective study of pregnant women infected with HBV, HBV reactivation rate was as high as 25.1%. As seen in this study, although reactivations are generally asymptomatic and recover spontaneously, some may be serious and can result in symptomatic hepatic decompensation. Most reactivations in this study were not in the postpartum period but in the intrapartum period, which was consistent with findings in literature. Therefore, women infected with HBV must be followed up closely during pregnancy and in the early postpartum period. As there is a limited number of studies in the literature, which have examined the risk factors for HBV reactivation in pregnancy, there is a need for further, prospective studies of larger cohorts to clarify this.