1. INTRODUCTION
Cardiac CT is an imaging modality capable of visualizing the anatomy of the heart and its surroundings (including great vessels and pericardial) [1]. Cardiac CT is used in clinical practice decades after the first CT scan is used to visualize the brain. The reason that it took so long for cardiac CT to be in its current position is the limitations of its early models, especially the number of slices acquired and its high radiation [2]. Nowadays, the technological advancement in cardiac CT gives better accuracy with lower radiation than its earlier models. Its entire procedure took a relatively short time and minimal contact. Cardiac CT is capable of acting as a triple rule out for acute chest pain in emergency settings. In non-emergency settings, Cardiac CT is known to assess the calcium score, and when used concomitantly with contrast, it gives a better visualization of the coronary arteries [2, 3]. In addition, studies have recommended cardiac CT use in excluding intracardiac thrombus before cardioversion and planning for cardiac intervention. However, developing countries have certain challenges and limitations in applicating the use of cardiac CT. This review aims to discuss the use of cardiac CT in managing cardiovascular diseases in clinical practice, explain the advantages of CCTA in emergency and non-emergency settings, as well as discuss its limitation so that we can make the best use of it, especially in developing countries.
2. METHODS
This writing is a literature review. Literature search using PubMed and Google Scholar database with keywords: Cardiac imaging techniques, coronary occlusion, CT angiography, developing countries, thrombosis. The inclusion criteria are articles related to the theme (all the article). Exclusion criteria were articles not related to the theme, and incomplete, such as an abstract-only article.
3. RESULTS AND DISCUSSION
3.1. EMERGENCY SETTING
3.1.1. CHEST PAIN TRIPLE RULE OUT (TRO)
Acute chest pain is one of the most common chief complaints in emergency settings [4]. Several guidelines have established a clear pathway for diagnosing patients with chest pain, especially those related to coronary artery diseases, but the same pathway may not be sufficient in diagnosing other causes of non-specific acute chest pain [5, 6]. The need for ruling cause of chest pain is to exclude life-threatening condition such as ACS, acute aortic disease, and PE to conditions that are relatively harmless. Patients who come to the emergency room with symptoms of acute chest pain will generally undergo ECG and cardiac biomarkers. Patients who have normal ECG results and negative troponins are generally discharged. However, it turns out that in some patients who experience ACS, ECG, and biomarker examinations can show normal results. To reduce the possibility of misdiagnosis, doctors are advised to observe for several hours and re-examine. Rapid and accurate diagnosis can help patients that in need of invasive coronary strategy, also in patient that in need of transfer to the catheterization laboratory [7, 8, 9]. CT angiography emerged as an imaging modality that can fill the gap. Multiple studies have proven the effectiveness of CT angiography acting as a TRO in evaluating the presence of acute coronary syndrome (ACS), aortic dissection, and pulmonary embolism (PE). In addition, study found that patients have shorter stay in ED and less exposed to radiation and contrast. The details of the studies using CCTA for patients with chest pain in the emergency department (ED) are provided in Table 1.
All studies mentioned in the table have consistently provided evidence that CT angiography done in the ED for patients with chest pain is beneficial. It was stated in the study conducted by Sawyer et al. that CCTA with TRO procedure was beneficial for the patient because the patient tends to have a shorter ED length of stay, is exposed to less radiation, and results in less cost [10]. Regarding the number of CT-scan slices for this procedure, the studies conducted by Dodd JD et al. and Branch et al. both used the fewest slice of CT (slices) compared to other studies. 64-slice CT-scan needs to be supported with Electrocardiogram (ECG)-gated imaging, contrast administration (both studies used triphasic contrast injection technique), and drug administration (both administered β-blocker to patients with high heart rate and nitroglycerine to all patients prior to the procedure). It was observed to be sufficient in picturing the coronary arteries and able to detect coronary obstruction in patients with chest pain [11, 12]. In addition to identifying and eliminating coronary blockage, the TRO method was intended to detect and exclude PE and aortic disorders. However, the 64-slice CT scan is reported to be poor in visualizing the pulmonary arteries (specifically the left main and left upper lobar arteries) and has minor motion artifacts in visualizing the aorta (specifically the aortic sinus and most caudal aspect) [12]. Thus, the image acquired should be interpreted cautiously in diagnosing PE or aortic diseases that occurred in the location mentioned.
Several trials have reported that CCTA, especially TRO protocol, has a very high sensitivity (ranging from 95% to 100%) and negative predictive value (ranging from 99% to 100%) for coronary obstruction [11, 13, 14]. These findings suggested that it could diagnose and rule out chest pain caused by coronary obstruction. This will let the clinicians suspect other causes of chest pain when there is no coronary lesion. Also, a study hospital with more CT-scan slice (128, 256, and 320 slices) have the same advantages as the 64-slice CT scan, which is highly effective in detecting and excluding coronary obstruction [15, 16, 17, 18]. In addition to that, the more CT-scan slice is more capable of detecting other causes of chest pain, such as PE and aortic diseases. Wnorowski diagnosed PE using a 256-slice CT scan that affected the main pulmonary arteries, lobar branches, and segmental branches (specifically upper lobes above the carina) [16].
Contrast administration has been one of the issues in using CCTA because it can cause contrast-induced nephropathy (CIN), even though permanent renal impairment because of contrast is rarely reported [19]. Cakmakci et al. conducted a study to tackle the issue by administering a low contrast dose. Among the studies included in the table, Cakmakci et al. administered the lowest amount of contrast. Furthermore, the low amount of contrast did not affect the quality of the procedure in detecting PE and aortic aneurysm. In conclusion, this procedure can be applied to patients in contrast agent-risk group [15]. The study was done with a 128-slice CT scan so that it can be inferred that a CT scan with a more slices can apply this technique to reduce contrast administration. On the other hand, further research needs to be done to examine the capability of the fewer slice CT-scan to use this technique.
Radiation exposure to the patient is another concern of CT-scan application in the clinical setting. 20 mSv/year is the maximum effective radiation dose that can be exposed to the patient to minimize the stochastic effect [20]. The mean radiation exposure from the six studies mentioned in the table was regulated under the maximum effective radiation dose, but it can be reduced further with technique modification and technological advancement. Tube current modulation and ECG-gated procedure played an essential role in reducing the amount of radiation exposure. Wnorowski showed the significant reduction in mean radiation exposure with tube current modulation in the study. The initial image acquisition without tube current modulation resulted in the highest mean radiation exposure among the studies. After the use of tube current modulation, the mean radiation exposure dropped approximately ten mSv to 8.75 mSv. Furthermore, another change in tube current modification (tube voltage of 100 kVp) lowered the mean radiation exposure to 5.3 mSv [16]. ECG-gated provided better imaging with a lower amount of radiation. The ECG-gated procedure can be divided into retrospective and prospective ECG-gated. Both provided an equivalent image quality, but the prospective ECG-gated procedure was reported to be better than the retrospective ECG-gated procedure in terms of radiation dose and aortic attenuation value [21].
Drug administration also played an important role not only in TRO procedures but also in cardiac CT in general. The ideal heart rate for the procedure is normal to slight bradycardia. Thus, when not contraindicated, the use of a β-blocker is crucial for image acquisition in a patient with tachycardia [22]. Metoprolol is the most common β-blocker administered to the patient because of its short half-life compared to other β-blockers [22, 23]. The indication, screening, and administration of β-blocker can be seen in a review conducted by Pannu et al. [23]. Nitroglycerine is another drug that is routinely administered sublingually to the patient before the CCTA procedure. In a systematic review of more than 217 studies, the administration of nitroglycerine increases the diagnostic accuracy of CCTA by dilating the coronary diameter, improving the number of coronary segments that can be accessed, and enhancing the image quality without major side effects reported [24]. Although beta-blockers are useful in lowering heart rate, they have a negative inotropic impact and may reduce left ventricular contractility. This may have an effect on assessing ventricular function; however, now, ventricular contractility is normally examined using echocardiography or nuclear medicine investigations, and CT's purpose is primarily to measure the coronary arteries [23].
In the emergency setting, CCTA with TRO procedure is vital to determine the unknown cause of chest pain as a part of managing patients with chest pain. The use of the TRO procedure becomes more relevant during the COVID-19 pandemic when diagnosis should be made with less contact with the patient. In addition, a study investigating the use of CCTA in a patient with chest pain during the COVID-19 era revealed that increased CCTA utilization was linked to a significantly shorter duration of stay with no increase in 30-day adverse outcomes. The 128-slice CCTA was used in that study; however, hospitals with a 64-slice CT scan can perform the TRO procedure because a 64-slice CT scan is the minimum requirement for this procedure [3, 25]. On the other hand, hospitals with CT-scan that have fewer slices than 64 cannot perform the TRO procedure. The alternative to this situation is to use a minimum 16-slice CT scan for CT angiography, or if referring possible, referral should be considered. Furthermore, since coronary artery motion artifact commonly inhibits exact evaluation at higher heart rates, a rapid heart rate (>65 beats/min) is a relative contraindication to TRO on 64-slice multi-detector CT. Newer scanners, such as the 128-slice dual-source CT (75 msec), have faster temporal resolution, which may make diagnostic imaging quality of the complete coronary arteries possible, even at heart rates of 75 beats/min and above. Finally, a high coronary artery calcium score (>1,000) might be a relative contraindication of TRO since significant calcification of the coronary arteries can hinder the assessment of coronary artery stenosis [26].
3.2. NON-EMERGENCY SETTING
3.2.1. CORONARY ARTERY CALCIUM (CAC) SCORING
Atherosclerotic cardiovascular disease (ASCVD) is one of the leading causes of death worldwide [27, 28]. The effort to reduce its incidence has been improving these several decades, from enhancing the curative approach to detecting high-risk patients so that lifestyle modification and initiation of lipid-lowering medication can be given as early as possible. The 2016 European guidelines on cardiovascular disease prevention in clinical practice and the 2019 American College of Cardiology/American Heart Association (ACC/AHA) guideline on the primary prevention of cardiovascular disease have suggested the risk stratification score that can be used in daily practice [29, 30]. The administration of statin in a patient with a low risk for ASCVD (< 5%) and high risk for ASCVD (≥ 20%) has been stated clearly in the algorithm. While for a patient with borderline risk (5% - <7.5%) and intermediate risk (≥ 7.5% - <20%), the benefit of statin administration should be determined due to its lifetime administration [30, 31]. The role of cardiac CT in determining CAC score becomes crucial in these groups in determining the decision which is favorable for the patient [32]. The most common method used is the Agatston method. The CAC score results from the multiplication between the lesion areas with the density factor. The density factor was determined using the max HU: 130-199 HU (factor 1), 200-299 HU (factor 2), 300-399 HU (factor 3), and ≥ 400 HU (factor 4). The result of the CAC score using the Agatston method can be divided into four groups: absent of coronary artery calcification with a score of 0, discrete coronary artery calcification with a score of 1-100, moderate coronary artery calcification with a score of 101-400, and accentuated coronary artery calcification with score >400 [33]. Patients with borderline or intermediate risk for ASCVD with CAC score 1-100 especially aged ≥ 55 years and any patient with CAC score ≥ 100 is indicated for statin therapy [34]. CAC score may also be a reference for statin initiation, while CAC density was known to act as the strongest independent predictor of major adverse cardiac events (MACE) in symptomatic patients undergoing CAC scans using CT angiography. The study used 320-slice CT scan to evaluate the coronary calcium of 379 symptomatic patients (suspected or known CAD). CAC density was found to be superior compared to the Agatston score, CAC volume, area, and mass. Thus, in the future, more studies can be conducted to further figure out the prognostic role of CAC density in symptomatic patients [35]. A meta-analysis that included 19 reports with 34,041 participants confirmed that CAC was independently associated with MACE in symptomatic patients suspected with CAD [36].
According to a recent study by Xia et al., in obese patients (BMI > 30 kg/m2), non-ECG-triggered chest CT is inadequately reliable for cardiovascular risk classification based on CAC scoring, and instead, an ECG-triggered cardiac CT is recommended [37].
For several years, multidetector computed tomography (MDCT) has replaced electron beam computed tomography (EBCT) as the first-choice modality in determining CAC score [33]. Modern MDCT resulted in lower radiation exposure (1 mSv) than traditional EBCT (2-3 mSv) which suits the utility of CT-scan as a screening examination [38]. Nonetheless, both CTs can still be used in clinical practice without contrast [39]. The use of ECG-gated CT improved the quality of the image acquired compared to the non-ECG-gated CT and was recommended by the guideline. However, its usage was challenging to be implemented because it needed specific software and additional hardware, and it may increase radiation exposure [32]. Thus, in clinical practice, the available imaging modality should be chosen to determine the CAC score/density because even though ECG-gated MDCT was recommended, the use of non-gated CT and EBCT is still possible. Unless referring the patient to the nearest hospital with a better imaging modality is possible.
3.2.2. RULING OUT LEFT ATRIAL APPENDAGE (LAA) THROMBUS PRIOR TO CARDIOVERSION
Cardioversion has been used widely to treat atrial fibrillation (AF) for several decades. It was used to treat both acute and persistent AF. In acute settings, immediate cardioversion can be done when the patient presents with hemodynamic instability or new-onset AF (< 48 hours). On the other hand, in patients with AF onset ≥ 48 hours, transesophageal echocardiography (TEE) needs to be done prior to cardioversion because of the risk of thromboembolic events [40]. In a real-world study, transient ischemic attack and non-hemorrhagic stroke occurred after the cardioversion even though the total prevalence of the two events was relatively small (0.6%) [41]. TEE is considered the gold standard for diagnosing LAA thrombus because of its high sensitivity and specificity (96% and 92%, respectively) [42]. However, 2-dimensional (2D) TEE has several limitations: it may misdiagnose thrombus <2 mm (which has a high risk of emboli), and it may not give a comprehensive evaluation of the complex structure of LAA [43]. A newer TEE, 3-dimensional (3D) TEE, was proven to be able to overcome the limitation possessed by the 2D TEE because it can clearly visualize the LAA morphology, especially pectinate muscle, which is often misdiagnosed as thrombus [44].
Cardiac CT has emerged as another imaging modality in assessing the presence of LAA thrombus. A meta-analysis conducted by Romero et al., which included 19 studies, found that the overall diagnostic accuracy of Cardiac CT in diagnosing LAA thrombus was 94%, with a sensitivity of 96% and specificity of 92%. The overall diagnostic accuracy increased to 99%, with a sensitivity of 100% and specificity of 99% when delayed imaging was done. Most of the CT used in the meta-analysis is 64 slices with/without ECG-gated CT-scan. It can be concluded from the meta-analysis that cardiac CT diagnostic accuracy with delayed imaging was slightly better than 3D TEE [42, 45]. Furthermore, cardiac CT can give a better image acquisition for LAA morphology. Previously, TEE was recommended as the gold standard for diagnosing LAA thrombus, and cardiac CT is used when TEE is contraindicated [45]. However, the use of cardiac CT has currently become more relevant than ever during the COVID-19 pandemic [46]. In addition to its excellent accuracy, which is similar to TEE, it is also a non-invasive procedure that causes fewer aerosols compared to TEE, a more comfortable procedure for the patient, and a more timely procedure [42, 45, 46]. Cardiac CT is preferred to TEE in diagnosing LAA thrombus prior to cardioversion in the latest ESC guideline [46].
A recent study developed an innovative algorithm for detecting an LAA thrombus by integrating two independent aspects of LAA enhancement by contrast media, the Hounsfield unit (HU) ratio and the standard deviation of HU density (HU-SD), which accurately indicated the absence or presence of an LAA thrombus in around 80% patients with persistent AF [47].
This shift of image acquisition modality priority might be the beginning of a mass adaptation in managing patients prior to cardioversion. Thus, two limitations of cardiac CT procedure should be taken into account before the procedure is held, which are the contrast administration and ionizing radiation [42].
3.2.3. CCTA IN PLANNING COMPREHENSIVE MANAGEMENT FOR THE PATIENT
More adoption of CCTA in clinical practice combined with its technological advancement led to another role for CCTA (besides diagnostic and prognostic imaging modality) which is pre-intervention planning imaging modality. CCTA can play an essential role in pre-intervention planning in at least two aspects. Firstly, CCTA can help cardiologists plan the comprehensive management of the patients, whether to administer fibrinolytic, refer the patient, or perform an invasive procedure. Secondly, the use of CCTA will help the interventionist to visualize and plan the intervention for a complicated case, especially for patients with chronic total occlusion (CTO). Therefore, when CCTA was done routinely before the intervention, several populations will be benefited: patients who did not have a coronary obstruction, patients who needed a bypass graft, and patients with a complicated case (chronic total occlusion). These benefits will be more relevant in developing country settings where cardiac interventionists and cardiothoracic surgeons are limited and unavailable in every health center.
Myocardial infarction with nonobstructive coronary arteries (MINOCA) was first reported more than 80 years ago, but the term is just recently used. MINOCA occurred in approximately 3.5-15% of AMI patients. The ranging prevalence was due to the higher occurrence in women and younger patients [48]. The cause of MINOCA can be classified as diseases in epicardial coronary arteries, microvascular, or both. The epicardial causes of MINOCA are coronary spasm, coronary dissection, and coronary plaque disruption, while microvascular causes of MINOCA are microvascular spasm, coronary thromboembolism, Takotsubo syndrome, and myocarditis [49]. CCTA is unable to detect the microvascular causes of MINOCA, but as mentioned earlier in triple rule out, CCTA has a high NPV for coronary occlusion. In these situations, CCTA done after the initial workup of the patient will reduce the invasive procedures done on the patient because revascularization is not a therapeutic option in patients with MINOCA [50]. The use of CCTA to exclude coronary occlusion is crucial during the COVID-19 pandemic, especially in patients that were infected with the virus [51]. COVID-19 was known to increase thrombosis, and thrombosis is one of the mechanisms underlying MINOCA [49, 52]. Cardiologists should also be aware of MINOCA in COVID-19 patients with acute chest pain and should exclude MINOCA before diagnosing myocarditis in COVID-19 patients [53].
ST-elevation myocardial infarction (STEMI) patients should be managed with fibrinolytic administration or primary percutaneous coronary intervention (PCI) [6]. As mentioned earlier, due to the limited cardiac interventionist/cardiothoracic surgeon in developing countries’ hospitals, administering fibrinolytic is the first attempt that can be made in all hospitals. However, if the use of fibrinolytic is contraindicated, a right referral should be made. The use of CCTA in the situation will be helpful in identifying patients with: a) left main LAD with intermediate-high synergy between percutaneous coronary intervention with TAXUS and cardiac surgery (SYNTAX) score; b) three-vessel CAD without diabetes with intermediate-high SYNTAX score; c) and three-vessel CAD with diabetes with low-high SYNTAX score who are more recommended to undergo coronary artery bypass grafting (CABG) than PCI [54]. When diagnosed with CCTA, the patient can be referred to the hospital by a cardiothoracic surgeon, and the patient can be planned to undergo CABG directly. On the other hand, patients with other coronary obstruction that is not included in the indication for CABG should be referred to an interventionist for PCI. The management algorithm for patients planning to undergo cardiac intervention can be seen in Figure 1.
3.2.4. CCTA IN PRE-INTERVENTION PLANNING FOR CHRONIC TOTAL OCLUSION (CTO)
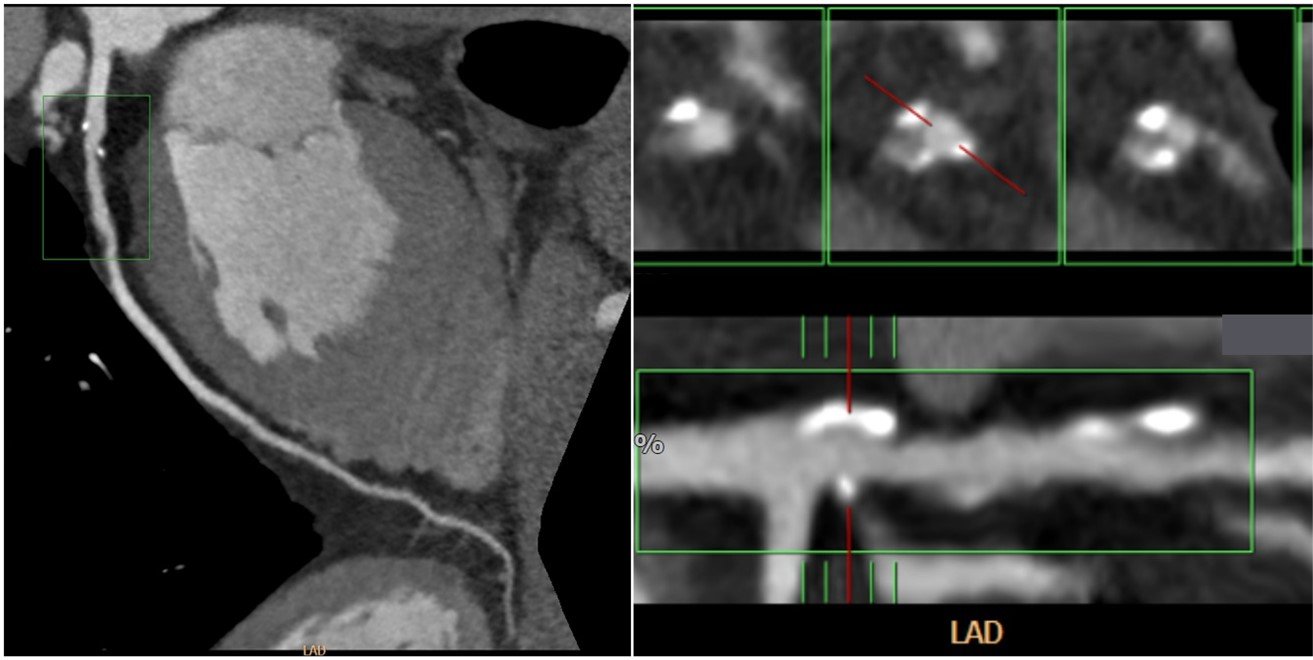
CTO is a complicated case that can be managed better with the help of CCTA. CCTA, in general, can help interventionists to visualize the length of the obstruction and the exact location of the obstruction. In managing CTO lesions, CCTA can prevent the complications of PCI, such as arterial dissection, perforation, and cardiac tamponade [55]. Two famous scorings, the Japanese Multicenter CTO Registry (J-CTO) and Computed Tomography Registry of Chronic Total Occlusion Revascularization (CT-RECTOR), were introduced to determine the difficulty levels of the intervention procedure objectively. J-CTO was introduced earlier by Morino et al. There are five components included in this scoring which are previously failed lesion, blunt stump type, bending, calcification, and occlusion length ≥ 20 mm. Each component is scored 1 point, and the accumulation of the points is classified into four groups: easy (score: 0), intermediate (score: 1), difficult (score: 2), and very difficult (score: ≥3) [56]. CT-RECTOR was introduced afterward by Opolski et al. Different from J-CTO, there are six components in CT-RECTOR: multiple occlusion, blunt stump within CTO, bending (≥45o) within CTO, the previous attempt of PCI, and occlusion duration ≥12 months or unknown. Similar to J-CTO, each component is scored 1 point, and the accumulation of points is divided into four groups with classification similar to J-CTO. Furthermore, the result of the CT-RECTOR score represents the difficulty of the intervention to be done in a more specific duration, ≤ 30 minutes [57]. CCTA prior to the cardiac intervention will provide important information regarding the lesion, especially with the help of the scoring system. This will enable the cardiac interventionist to have more preparation for the intervention. According to a recent study, pre-procedural CCTA for CTO led to greater success rates and statistically less acute periprocedural complications than angiography guidance, namely coronary perforations, and periprocedural myocardial infarction. In addition, patients with CTO who had a high J-CTO score showed greater success rates than those who did not [58].
Calcification is one of the major concerns in the invasive procedure because there are several negative consequences regarding plaque with calcification: failure to deliver the stent, malposition, and not maximally expanded [59]. These consequences can lead to re-intervention/surgery so it should be prevented. Invasive coronary angiography (ICA) was found not fully capable of diagnosing calcified lesions with milder degrees, and intravascular ultrasound may increase the capability of ICA in detecting the calcified lesion only up until >70% of lesions [59, 60]. On the other hand, CCTA was excellent in diagnosing coronary artery stenosis with a calcified plaque in type I-II calcified plaque, but it needs to be assisted by the CAC score in type III-IV calcified plaque [61]. Furthermore, CCTA can provide 2D and 3D data according to the CT scan used, while ICA can only provide 2D data. Therefore, intervention planned with CCTA, especially with 3D CCTA, can give a more exact situation and anatomy of the obstruction. The study conducted by Li et al. confirmed it with their finding that 3D CCTA is significantly better in discriminating ischemia in patients suspected of CAD compared to ICA (p<0.01) [62].
4. LIMITATIONS AND THE FUTURE OF CCTA
The limitation of performing CCTA prior to the intervention is the time taken to perform the image-acquiring procedure that took approximately 10-20 minutes, which will delay the door-to-needle or door-to-balloon time. Another limitation of this procedure occurs in those with renal function impairment because these patients are contraindicated for contrast administration, which is necessary for this procedure. These challenges need to be solved in the future with more advancements in technology and should be supported by the evidence provided in future studies. There are also several limitations implementing this procedure, especially in developing countries with limited equipment and human resources that are primarily available in large hospitals. The reimbursement of this procedure also becomes a limitation in developed countries and even more in developing countries.
However, Image quality is also one of the drawbacks of CCTA. Image quality is limited by patient conditions, such as obesity, dense calcifications, multiple or small diameter stents, high heart rates, and non-sinus rhythm [63]. A necessity for ED physicians and cardiologists to have in-depth knowledge of the applications and limitations of CCTA. A cardiologist and radiologist must carry out a complete assessment of the results from the CCTA examination where not all hospital emergency departments have it.
Fractional flow reserve (FFR) is widely used with PCI to determine lesions that are functionally impaired to be targeted for intervention. FFR technology is now starting to be applied in CT-scan as FFRCT [59, 64]. The proportion of maximum blood flow that persists despite the stenosis is what the invasive FFR measures and it is defined as the ratio of the mean distal coronary pressure to the mean aortic pressure during hyperemia [65]. Revascularization treatments guided by FFR have been found to decrease the incidence of Major adverse cardiovascular events and costs. Recent computational flow dynamics (CFD) advancements have allowed for the non-invasive diagnosis of hemodynamically significant stenosis utilizing CT data alone. CFD techniques are used to model coronary blood flow and acquire functional information with FFR-CT [66]. Several trials (DISCCOVER-FLOW, DeFACTO study, NXT trial, Platform study, ADVANCE registry, and CHINA CT-FFR trial) have stated the benefits of using FFRCT [59, 64]. However, the availability of FFRCT is still scarce in developing countries. In developing countries, FFRCT can become an even better pre-intervention imaging modality in the future.
CT perfusion imaging of the cardiac is a relatively new clinical imaging modality that allows for visualization and quantification of iodinated contrast material inside the cardiac at rest and during pharmacologic stress. According to the literature, the sensitivity and specificity of CTP are between 71% and 95%, whereas those of invasive FFR is between 84% and 95% [67]. Both static and dynamic CTP may suffer from diminished diagnostic picture quality due to the presence of beam hardening artifacts; nevertheless, multiphase acquisitions mainly give the chance to pick images with diminished (beam hardening) artifacts, even though dynamic imaging is more susceptible to motion artifacts in general. Dynamic CTP has a number of disadvantages, including the increased radiation dosage required for conventional CT settings, more complex acquisition processes, and advanced analytical techniques [68].
Advanced coronary plaque analysis with CCTA can be done using qualitative or semiquantitative methods to evaluate a single lesion or more thorough methods that can quantify the volume of atherosclerosis in a patient. The coronary artery luminal area, atherosclerotic plaque area, and plaque volume can now be accurately quantified using postprocessing tools [69]. However, it has been noted that variations in scan parameters (Kvp and mA) and variations in coronary lumen contrast attenuation may affect interscan consistency of plaque subtype characterization [70]. Beyond lumen stenosis severity, CCTA advanced atherosclerosis analysis has been shown to have a significant prognostic utility in a number of studies. Plaque subcomponent quantification may be influenced by age and sex, as was recently found in a subanalysis of the ICONIC research [71]. Specifically, the calcified plaque volume of a patient rises with age in both male and female subjects, while the noncalcified plaque volume is lower in females across all ages. However, there were no significant differences in the incidence of qualitative high-risk plaque characteristics or necrotic core volume by either age or sex. These insights must be considered for a more accurate interpretation of advanced plaque analysis [66].
5. CONCLUSIONS
Cardiac CT advancement enables it to become an important imaging modality in clinical practice during emergency and non-emergency settings and in planning comprehensive management of cardiac patients. In emergency settings, cardiac CT as TRO is useful to exclude life-threatening conditions such as ACS, acute aortic disease, and PE for conditions that are relatively harmless. This really helps patients, as this is used as a modality in planning comprehensive management of cardiac patients, especially CCTA in planning for cardiac intervention as soon as possible. This also reduces the duration of patient care in the emergency room so that treatment costs can be efficient. Cardiac CT in non-emergency settings is capable of determining CAC score to initiate statin administration in patients with borderline and intermediate-risk and detecting LAA thrombus prior to cardioversion. This imaging modality becomes more relevant to be used in clinical practice during the pandemic due to less contact with the patient needed during the procedure, and physicians may get used to using cardiac CT. Developing countries have several limitations in applying cardiac CT in clinical practice, including limited equipment, human resources, and reimbursement.