Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Anales de Psicología
versión On-line ISSN 1695-2294versión impresa ISSN 0212-9728
Anal. Psicol. vol.29 no.1 Murcia ene. 2013
https://dx.doi.org/10.6018/analesps.29.1.138231
Frontal variant of Alzheimer's disease and typical Alzheimer's disease: A comparative study
La variante frontal de la enfermedad de Alzheimer y la enfermedad de Alzheimer típica: un estudio comparativo
Bernardino Fernández-Calvo1, Francisco Ramos2 Virginia Menezes de Lucena3
1 Department of Psychology. CCHLA. Federal University of Paraiba, Brasil
2 Department of Personality, Assessment and Psychological Treatments. University of Salamanca, Spain
3 Department of Internal Medicine. CCM. Federal University of Paraiba, Brasil
The study was supported in part by Velum foundation.
RESUMEN
Una de las características de la Enfermedad de Alzheimer (EA) es su heterogeneidad clínica. Así, la presentación atípica frontal o disejecutiva es cada vez más conocida, aunque los factores subyacentes se desconocen. En este estudio se comparó el rendimiento neuropsicológico de dos grupos de pacientes con EA (variante frontal -EAvf- y típica -EAT-). El grupo EAvf (n = 13) fue seleccionado por la existencia de una hipocaptación frontal. Los resultados revelaron que el grupo EAvf manifestó un trastorno disejecutivo grave, una sintomatología neuropsiquiátrica más severa (desinhibición y apatía), mayor deterioro funcional y generó mayor sobrecarga en el cuidador que el grupo EAT sin afectación frontal (n = 47). A pesar de que el grupo EAvf rindió más bajo en todos los test neuropsicológicos, solo se encontraron diferencias significativas entre ambos grupos en las tareas de velocidad de procesamiento y visuoconstrucción. El análisis de regresión logística reveló que las puntuaciones de velocidad de procesamiento y flexibilidad mental predicen significativamente el diagnostico de EAvf. La existencia de reflejo de graspin, anosognosia y la no posesión del APOE e4 también fue más prevalente en el grupo EAvf. Este grupo mostró una predominancia de varones y fue más propenso a tener una historia familiar positiva para la EA. Para concluir, el estudio sugiere que la EAvf representa un subtipo de EA que parece tener características clínicas, neuropsicológicas y genéticas diferentes a la EAT.
Palabras clave: Variante frontal de la enfermedad de Alzheimer; funciones ejecutivas; anosognosia; Apolipoprotein E; déficits neuropsicológicos; trastornos neuropsiquiátricos.
ABSTRACT
Clinical heterogeneity is one of the characteristics of Alzheimer's disease (AD). Hence, the atypical frontal or dysexecutive presentation is becoming increasingly well-known, although the underlying factors are still unknown. In this study, the neuropsychological performance of two groups of patients with AD (frontal variant--ADfv--and typical--TAD) were compared. The ADfv group (n = 13) was selected due to the existence of frontal hypoperfusion on a simple photon emission computer tomography (SPECT). The results revealed that the ADfv group displayed a severe dysexecutive disorder, more severe neuropsychiatric symptomatology (disinhibition and apathy), more functional impairment, and it generated a higher caregiver overload than the TAD group without frontal impairment (n = 47). Despite the facts that the ADfv group's performance was poorer in all the neuropsychological tests, significant group differences were only found in the processing speed and visuoconstruction tasks. Logistic regression analysis revealed that the processing speed and mental flexibility scores significantly predicted a diagnosis of ADfv. The existence of the grasp reflex, anosognosia, and the absence of apolipoprotein E epsilon 4 allele (APOE e4) were also more prevalent in the ADfv group. This group had a predominance of males and it was more likely to have a positive family history of AD. To conclude, the study suggests that ADfv represents a subtype of AD that seems to have different clinical, neuropsychological, and genetic characteristics from TAD.
Key words: Frontal variant Alzheimer's disease; executive functions; anosognosia; Apolipoprotein E; neuropsychological deficits; neuropsychiatric disorders.
Introduction
The typical presentation of Alzheimer's disease (AD) implies a deterioration of episodic memory, with less severe deficits in attention, semantic memory, and visuospatial skills (Perri, Watson & Hodges, 2000), which is related to initial neuropathological lesions in the medial temporal lobe (entorhinal cortex and hippocampal formation), subsequently extending to other regions associated with the neocortex (Braak & Braak, 1991).
However, some authors suggest that impairment of the executive functions (EF) (Lafleche & Albert, 1995) and the neuropsychiatric alterations (Mega, Lee, Dinov, Mishkin, Toga & Cummings, 2000) can also be early characteristics of AD, with high prognostic value (Chen, Sultzer, Hinkin, Mahler & Cummings, 1998; Palmer et al., 2011). In this context, other profiles of cognitive impairment (Grady et al., 1988; Stopford, Snowden, Thompson & Neary, 2008), with a different evolution (Galton, Patterson, Xuereb & Hodges, 2000; Lambon, Patterson, Graham, Dawson & Hodges, 2003) have been described.
This heterogeneity of AD was revealed in the results of a series of neuropathological (Galton et al., 2000; Kanne, Balota, Storandt, McKeel & Morris, 1998), neuropsychological (Becker, Huff, Nebes, Holland & Boller, 1988; Stopford et al., 2008), and neuroimaging (Grady et al., 1990) studies that established the existence of three subtypes or atypical presentations of AD: visual agnosic, aphasic and apraxic (Wallin & Blennow, 1996). These subtypes are closely related to the location, degree, and type of neuropathology present in each one (Galton et al., 2000; Kanne et al., 1998; Lambon et al., 2003).
In the last decade, another subtype of AD has been differentiated and referenced. Some neuroimaging studies confirm the presence of a subgroup of patients with early AD who display frontal and temporal-parietal hypometabolism with neuropsychiatric (Chase, Burrows & Mohr, 1987; Grady et al., 1990; Perani et al., 1988) and dysexecutive disorders (Mann, Mohr, Gearing & Chase, 1992). Johnson, Head, Kim, Starr & Cotman (1999) detected a subtype of patients, which they called a frontal variant of AD (ADfv), which correlates neuropathologically with a higher number of neurofibrillary tangles (NTFs) in the frontal lobe, and excessive dysexecutive deficit (e.g., Trail Making Test-A [TMT-A] and FAS verbal fluency test [FAS]) in comparison to other patients with typical AD (TAD). Subsequently, this executive subgroup of AD has also been reported in a series of clinical cases (Jeong, 2003; Larner, 2006), clinical-anatomo-pathological descriptions (Alladi et al., 2007; Habek, Hajnsek, Zarkovic, Chudy & Mubrin, 2010; Taylor, Probst, Miserez, Monsch & Tolnay, 2008), and neuropsychological studies (Back-Madruga et al., 2002; Binetti, Magni, Padovani, Cappa, Bianchetti & Trabucchi, 1996; Woodward et al., 2010a).
With regard to patients with TAD, neuropsychological findings show that people affected with ADfv are slower to process information (Back-Madruga et al., 2002), their functional performance is poorer (Back-Madruga et al., 2002; Swanberg, Tractenberg, Mohs, Thal & Cummings, 2004; Woodward et al., 2010a), their global impairment progresses faster (Woodward et al., 2010a), and they generate more caregiver overload (Back-Madruga et al., 2002). It is important to note that these signs do not correlate with the severity of the dementia. Moreover, these patients do not usually present positive family history of dementia, and their symptoms are clinically (Woodward et al., 2010b), but not neuropsychologically, more similar (Woodward et al., 2010a) to frontotemporal dementia (FTD).
However, the neuropsychological identification of this ADfv group has not yet been confirmed by means of neuroimaging tests, so these clinical-neuropsychological results should be taken with precaution. Therefore, some of these neuropsychological findings may not coincide with the neuropsychological data contributed by publications of clinical-neuropathological descriptions of patients with ADfv (Habek et al., 2010; Taylor et al., 2008). In this sense, more research is needed of people affected with ADfv, defined through neuroimaging studies that confirm the neuroanatomic connection of the executive deficits, in order to analyze these and other neuropsychological characteristics, or features of another nature (e.g., genetic - the presence of apolipoprotein E epsilon 4 allele (APOE e4). A better description of ADfv could help to differentiate it from TAD and FTD. In this sense, some authors note that certain patients affected with neuropathologically confirmed AD, and who present executive deficits and neuropsychiatric disorders in addition to amnesia, are diagnosed with FTD (Alladi et al., 2007; Brun, 1987) or AD (Balasa et al., 2011) at the onset of the clinical picture; a diagnosis that is maintained until their deaths despite their manifesting, in the case of FTD, clear mnestic disorders a few years after the diagnosis (Alladi et al., 2007). Whereas other works suggest that, in the atypical presentations of AD, there is a low prevalence of APOE e4 alíele (Snowden et al., 2007) and that AD patients who are not APOE s4 carriers present greater executive déficits (Wolk et al, 2010).
Thus, this investigation attempts to compare the neuro-psychological performance of the ADfv group, defined on the basis of frontal hypoperfusion, and the TAD group (without frontal hypoperfusion), also analyzing the relation between these two groups of patients with the APOE e4 allele. First, we hypothesize that the ADfv group will display more executive deficits than the TAD group. Second, we hypothesize that both groups of participants will achieve a similar neuropsychological performance in other non-executive tests. However, we expect to find group differences in the frequency of the APOE e4 allele. Specifically, the ADfv group will present a lower frequency of the APOE e4 allele.
Method
Participants
The 84 participants in the study, 60 patients and 24 cognitively healthy participants (CH), were selected from the Neurology Section of the University Hospital of Salamanca and from the family caregivers association of patients with Alzheimer's disease (AFA) of Salamanca, respectively. The data of the patients were collected consecutively from July to December 2002. In contrast, the data of the CH group were collected from January to April of 2003. The CHs and the person in charge of each patient gave their informed consent to participate in the study.
The patients underwent a general clinical, neurological, and neuropsychiatric examination, as well as the following complementary studies: hemogram, Homocysteine, general biochemistry, T4-TSH, vitamin B12 and folic acid, luetic serology, APOE, a neuroimaging study: computed tomography (CT) or magnetic resonance imaging (MRI) and simple photon emission computer tomography (SPECT), using the radiopharmaceutic 99mTc-hexamethylpropyleneamine oxime (Tc99m-HMPAO). The assessment of each brain SPECT was carried out visually by a radiologist who was blind to the patients' clinical diagnosis, and who classified the perfusion deficits according to the criteria proposed by Holman et al. (Holman, Johnson, Gerada, Carvalho & Satlin, 1992).
The diagnosis of AD was made according to the criteria of the National Institute of Neurologic, Communicative Disorders and Stroke - Alzheimer's Disease and Related Disorders Association (McKhann, Drachman, Folstein, Katzman, Price & Stadlan, 1984). The diagnosis was supported by a neuropsychological assessment using screening tests and a neuropsychological battery developed to assess diverse cognitive functions (memory, language, praxis, visuospatial function, attention, and executive functions), which has been shown to be efficacious to diagnose dementia (Contador, Fernandez-Calvo, Cacho, Ramos & Hernández-Martín, 2009). Only patients who met the criteria of "probable" Alzheimer-type dementia and who had a mild degree of dementia (CDR-1) according to the Clinical Dementia Rating (Hughes, Berg, Danziger, Coben & Martin, 1982) were included. All the patients were exempt from signs of focal deficit in the neurological exploration--less than four points on the Hachinski scale (Hachinski, et al., 1975)--and from focal lesions in the CT or the MRI of the brain. None of them met the criteria for major depression according to the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (American Psychiatric Association, 1994).
The patients with AD were classified according to the result of the brain SPECT as: frontal variant AD (ADfv, n = 13) or typical AD (TAD, n = 47). The pattern of hypoperfusion found in the patients was: normal (3), frontal (13), unilateral temporal (14), and bilateral temporoparietal (30).
Lastly, during the clinical interview, we confirmed the level of awareness of their own cognitive deficits, using a semi-quantitative scale developed by Reed, Jagust & Coulter (1993), the presence of positive family history of AD, defined as having at least one biological parent with AD, extrapyramidal signs (rigidity, tremor, bradykinesia), and grasp reflex. In order to guarantee the diagnosis, each patient with AD was followed up for at least five years to confirm the AD diagnosis.
Control participants. The 24 CH participants were healthy older Spanish-speaking people, who lived independently, were not institutionalized, and who maintained their daily activities, both basic and instrumental, integrally. People who presented a history of psychiatric or neurological disease, or alcoholism, or who were under psychopharmacological treatment and who had a score equal to or higher than 27 in the Mini Mental State Examination (MMSE) were excluded from the sample (Folstein, Folstein & McHugh, 1975). All the participants from the CH group performed the same psychometric and neuropsychological tests and in the same sequence as the group of patients.
Measures
All the participants were assessed by means of a broad neuropsychological battery that explored diverse cognitive functions, neuropsychiatric symptoms, and functional capacity: Processing speed: Trail Making Test-part A (TMT-A; Strauss, Sherman & Spreen, 2006); Digit Symbol Substitution subtests (DSST) of the Wechsler Adult Intelligence Scale-revised (WAIS-R; Wechsler, 1981); Attention: Forward digit span subtests (FDS) of the WAIS-R (Wechsler, 1981); A-Random Letter Test of Auditory Vigilance (A-Test; Strub & Black, 2000); Working memory: Backward digit span sub-tests (BDS) of the WAIS-R (Wechsler, 1981); Verbal memory: Hopkins Verbal Learning Test-RA (HVLT-RA; Benedict, Schretlen, Groninger & Brandt, 1998); Visual memory: Benton Visual Retention Test (BVRT; Benton, 1981); Rey-Osterreith Complex Figure (ROCF), delayed reproduction (Rey, 1987); Semantic memory: animal fluency test (AF; Strauss, et al., 2006); Visuoconstruction: Clock drawing test copy condition (CDT-C; Cacho et al., 2005); copy of the ROCF (Rey, 1987); Executive functioning: TMT-B (Strauss, et al., 2006), Stroop test (part C) (Strauss et al., 2006), FAS (Strauss et al., 2006); Similarities subtest (S) and Comprehension subtest (C) of the WAIS-R (Wechsler, 1981); Performance strategies of the ROCF (Rey, 1987); Neuropsychiatric Alteration : Neuropsychiatric Inventory (NPI; Vilalta-Franch, Lozano, Hernández, Llinás, López-Pousa & López, 1999); Functional Alterations: Interview for Deterioration in Daily Living Activities in Dementia (IDDD; Böhm, Peña-Casanova, Aguilar, Hernández, Sol & Blesa, 1998); Caregiver burden: Zarit Burden Interview (ZBI; Izal & Montorio, 1994).
Statistical analyses
The statistical analysis of the data was carried out with the software of the Statistical Package for Social Sciences (SPSS) version 17. The χ2 contingency test was used for dichotomous variables. Group differences in the continuous variables were analyzed with nonparametric tests (the Mann-Whitney U or the Kruskal-Wallis H), as the groups had a small and unequal n. Subsequently, multiple comparisons were examined with Dunn's test. Lastly, logistic regression analysis was used to determine the scores of the neuropsychological tests that were capable of predicting the presence or absence of ADfv. The level of significance was p < .05.
Results
Table 1 shows the participants' sociodemographic and clinical characteristics. In comparison to the TAD group, the ADfv group was made up predominantly of males, was more likely to have a positive family history of AD, displaying significantly higher frequency of anosognosia, grasp reflex, and a lower proportion of the APOE e4 allele. Like-wise, the ADfv group presented a significant increase in functional and neuropsychiatric impairment and generated greater caregiver overload, despite the fact that both groups of patients were balanced in their degree of impairment and years of evolution of symptomatology (TAD, range 1 to 4.5 years; ADfv, range 1.5 to 4.0 years).
The executive functioning of the ADfv group was also significantly lower in all five executive tests administered (Table 2). In view of these differences, we decided to determine the number of patients of each group with significantly deteriorated scores in the executive tests, which consisted of displaying < 2SDs from the CH group's mean in each measure, after standardizing by age and schooling.
A greater number of patients from the ADfv group had significantly worse scores in the TMT-B-, Stroop C, and FAS measures. In two other executive measures, there were also a higher number of ADfv patients who performed worse than the patients from the TAD group, although the difference was marginal. Moreover, 11 of these 13 patients from the ADfv group presented significantly worse scores in at least 4 out of the 5 executive tests administered. In contrast, none of the TAD patients obtained deteriorated scores in more than two executive tests (Table 3). Additionally, the ADfv group employed worse production strategies when copying the ROCF than the TAD group (χ2 = 20.4, p <.001).
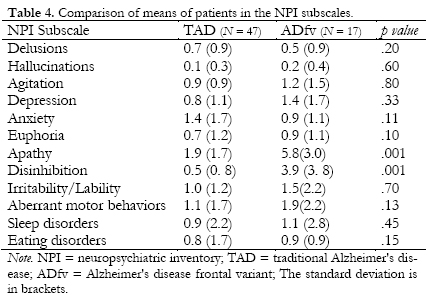
The patients from the ADfv group displayed more apathy, disinhibition, and worse processing speed and visuoconstruction than the TAD group (see Tables 4 and 5). It is important to note that the performance of the ADfv group was lower in all the cognitive functions analyzed. In fact, in some of them, we found marginal significance, such as the case of immediate and delayed visual memory.
Logistic Regression Analysis
In the logistic regression analysis, we entered the scores of the tests that were lower in the ADfv patients than in the TAD patients (TMT-A, DSST, CDT-C, ROCF-DR, TMT-B, Stroop- C, S, C, FAS). The model was significant (χ2 = 13.3, p < .001); the TMT-B tests [Exp(B) = .97, CI: (.95-.99), p = .02], FAS [Exp(B) = .78, CI: (.64-.94), p = .01], and TMT-A [Exp(B) = .96, CI: (.95-.99), p = .01] significantly predicted the presence of ADfv, correctly classifying 82% of the patients (69% ADfv and 85% AD).
Discussion
This work reveals the existence of a subgroup of AD with frontal hypoperfusion that differs from the rest of the patients with AD in a series of neuropsychological and genetic characteristics. Moreover, we found that these differences also include the clinical sphere. Our results show that dysexecutive impairment is more pronounced and homogeneous in the ADfv subgroup than in the TAD group, to the extent that approximately 77% of the participants of the ADfv group displayed severe impairment in the five executive measures analyzed in the study. The other 13% showed impairment in at least three executive tests. In contrast, 47% of the patients of the TAD group scored within the normal range in all the tests, whereas the rest of the patients showed impairment in just one measure, and there was more heterogeneity in the deteriorated test.
Therefore, we can consider that the existence of a dysexecutive disorder (< 2 SD) at the onset of the disease seems characteristic of a subgroup of patients with atypical dysexecutive presentation of AD. Other neuropathological and neuropsychological studies (Back-Madruga et al., 2002; Stopford et al., 2008; Woodward et al., 2010a; Woodward et al., 2010b) have observed this type of atypical presentation with early impairment of the frontal lobe in AD. For example, Stopford et al. (2008) found that 22% of the patients studied presented impairment in a nonamnestic single domain (language, visuospatial and praxis), and the dysexecutive presentation was the most frequent (9%). The patients of this group were more prone to display frontal hypoperfusion than the other patients. In contrast, patients with aphasic presentation did not display frontal alterations in the SPECT. Other neuroimaging studies (Grady et al., 1988; Perani et al., 1988) also detected the presence of a subgroup of AD with frontal hypoperfusion that coexists with bilateral temporal and parietal dysfunction, but they did not use a neuropsychological criterion to analyze the degree of impairment of the executive deficits nor did they carry out a comparative study with another type of patients with TAD. To our knowledge, this investigation is the first work that combined neuroimaging and a neuropsychological method to define and analyze the ADfv group.
Furthermore, the ADfv group presents an increase in the neuropsychiatric symptomatology, greater functional impairment, and it generates greater caregiver overload than the TAD group. On the one hand, these results confirm the findings of other neuropsychological studies (Back-Madruga et al., 2002; Woodward et al., 2010a), clinical (Larner, 2006; Woodward et al., 2010b) and/or neuropathological (Habek et al., 2010; Taylor et al., 2008); on the other hand, they show the relation between dysexecutive disorder and the presence of functional and behavioral impairments in early AD (Chen et al., 1998), verifying that the increase in the neuropsychiatric symptoms of AD (e.g., disinhibition) is significantly related to greater impairment of the frontal lobe (Bruen, McGeown, Shanks & Venneri, 2008; Mega et al., 2000) and to caregiver overload (Mohamed, Rosenheck, Lyketsos & Schneider, 2010). In this sense, caregivers of other kinds of non-Alzheimer dementias show higher rates of overload than caregivers of patients with AD (Ricci et al., 2009).
However, the performance of the ADfv group in other neuropsychological skills is similar to that of the TAD group, except for their greater impairment in processing speed and visuoconstruction, with worse planning strategies. This result partially coincides with that of Back-Madruga et al. (2002) and corroborates the neuropsychological descriptions carried out in the series of clinical cases with this variant of AD (Jeong et al., 2003; Johnson et al., 1999; Taylor et al., 2008).
Lastly, our ADfv group presented a higher frequency of grasp reflex and anosognosia, and lower presence of the APOE e4 allele. The existence of anosognosia in the ADfv group has also been described in the work of Taylor et al. (2008). Moreover, Starkstein, Jorge, Mizrahi, Adrian & Robinson (2007) suggest that the people affected by AD with greater frontal impairment may develop anosognosia even at initial stages of the disease, as our results indicate. Amanzio et al. (2011) consider that the impairment in cognitive flexibility may be a prerequisite for anosognosia in AD. In our study, cognitive flexibility, measured with the TMT-B and the FAS, is the executive component that undergoes the greatest alteration in the ADfv group, and less in the TAD group, and it significantly predicts, along with the TMT-A score, the diagnosis in 69% of the cases of ADfv and it excluded the diagnosis in 85% of the cases of TAD.
However, we consider that the existence of greater apathy and disinhibition in the ADfv group has to do with the neuroanatomic relation (e.g., dorsolateral frontal and anterior cingulate cortex) that seems to exist between these symptoms, executive functioning and anosognosia (Amanzio et al., 2011; Starkstein, Brockman, Bruce & Petracca, 2010). In this sense, the present results confirm the opinion of some authors who state that flexible thinking and the ability to inhibit a response seem to be essential skills to become aware of the cognitive deficits in the daily life of patients with AD (Amanzio et al., 2011). Likewise, the most severe visuoconstructive deficits of patients with focal brain damage are related to anosognosia and the impairment of the dorsolateral prefrontal cortex (Rinaldi, Piras & Pizzamiglio, 2010). In fact, Fink, et al. (1999) indicate that these structures are essential to monitor the congruence between one's expectations and what is actually done when carrying out goal-directed actions. It is feasible that an impairment of these frontal structures, which generates incapacity to detect the incoherence between a foreseen action and the action performed when copying or reproducing models (Rinaldi et al., 2010), may have caused more severe visuoconstructive deficits in the ADfv group, compared to the TAD group.
Some authors relate the absence of the APOE e4 allele in AD to a greater atrophy in the frontoparietal structures (e.g., dorsofrontal-parietal atrophy; Wolk et al., 2010). Thus, the noncarriers of APOE e4 allele present greater impairment of the executive functions (Wolk et al., 2010). In any event, it is noted that other atypical presentations of AD have also been associated with a low prevalence of APOE e4, which suggests that this gene may not be very relevant as a risk factor for atypical phenotypes (Snowden et al., 2007).
This study also has some limitations. First, the dysexecutive disorder presented by the patients with AD may be secondary to white matter hyperintensities in the fronto-subcortical circuits. However, this seems improbable; we included the criterion of scoring < 4 on a Hachinski ischemic scale, which reduces the significant presence of vascular disease. Second, it is possible that the participants of the ADfv group presented frontotemporal dementia (FTD) instead of AD, because of their notable neuropsychiatric and dysexecutive disorder. Nevertheless, this seems improbable for two reasons. On the one hand, the mean age at the onset of the disease in the ADfv group was relatively higher (72 years) than the age generally observed in FTD (onset before 65 years). On the other hand, the ADfv group showed a visuospatial deficit; a skill that is relatively well preserved in the initial phase of frontotemporal dementia (Hutchinson & Mathias, 2007). Lastly, there was no neuropathological confirmation of the clinical diagnoses presented in this study.
Conclusions
Despite these limitations, the results of the study show the clinical heterogeneity of early AD by detecting a subgroup of AD, called ADfv, with phenotypical clinical and neuropsychological characteristics that are different from those of TAD; characteristics that, in the absence of neuropathological confirmation, seem to be associated with a variety of risk factors and genes, and not merely with APOE. Although ADfv is a rare atypical presentation (Wallin & Blennow, 1996), it is necessary to carry out other studies in order to advance in the phenotypical and neuropathological description of this variant of AD. This information may help to homogenize patients for pharmacological investigation and facilitate clinical practice, improving the differential and prognostic diagnosis among the diverse clinical phenotypes of AD and other non-Alzheimer dementias (e.g., fronto-temporal dementia).
Acknowledgements
We thank all the participants for their time and commitment to the study. The authors also thank Dr. Israel Contador for his very helpful comments on this paper.
References
1. Alladi, S., Xuereb, J., Bak, T., Nestor, P., Knibb, J., Patterson, K., & Hodges, J. R. (2007). Focal cortical presentations of Alzheimer's disease. Brain, 130, 2636-2645. [ Links ]
2. Amanzio, M., Torta, D. M. E., Sacco, K., Cauda, F., D' Agata, F., Duca, S., Germmani, G. C. (2011). Un awareness of deficits in Alzheimer' s disease: role of the cingulate cortex. Brain, 134, 1061-1076. [ Links ]
3. American Psychiatric Association (1994). Diagnostic and statistical manual of mental disorders: DSM-IV-TR (4th ed.). Washington, DC: American Psychiatric Press. [ Links ]
4. Back-Madruga, C., Boone, K. B, Briere, J., Cummings, J., McPherson, S., Fairbanks, L., & Thompson, E. (2002). Functional ability in executive variant Alzheimer's disease and typical Alzheimer's disease. The Clinical Neuropsychologist, 16, 331-340. [ Links ]
5. Balasa, M., Gelpi, E., Antonell, A., Rey, M. J., Sánchez-Valle, R., Molinuevo, J. L., & Lladó, A. (2011). Clinical features and APOE genotype of pathologically proven early-onset Alzheimer disease. Neurology, 76(20), 1720-1725. [ Links ]
6. Becker, J. T., Huff, F. J., Nebes, R. D., Holland, A. & Boller, F., (1988). Neuropsychological function in Alzheimer's disease: pattern of impairment and rates of progression. Archives of Neurology, 45, 263-268. [ Links ]
7. Benton, A. L. (1981). El Test de Retención Visual. Ediciones TEA. Madrid. [ Links ]
8. Binetti, G., Magni, E., Padovani, A., Cappa, S. F., Bianchetti, A., & Trabucchi, M. (1996). Executive dysfunction in early Alzheimer's disease. Journal of Neurology, Neurosurgery, and Psychiatry, 60, 91-93. [ Links ]
9. Böhm, P., Peña-Casanova, J., Aguilar, M., Hernández, G., Sol, J. M., & Blesa, R. (1998). Clinical validity and utility of the Interview for Deterioration of Daily Living in Dementia for Spanish-speaking communities. International Psychogeriatric, 10, 261-270. [ Links ]
10. Braak, H., & Braak, E. (1991). Neuropathological staging of Alzheimer-related changes. Acta Neuropathologica, 82, 239-259. [ Links ]
11. Bruen, P. D., McGeown, W. J., Shanks, M. F., & Venneri, A. (2008). Neuroanatomical correlates of neuropsychiatric symptoms in Alzheimer' s disease. Brain. 131, 2455-2463. [ Links ]
12. Brun, A. (1987). Frontal lobe degeneration of non-Alzheimer type. I. Neuropathology. Archives of Gerontology Geriatric, 6, 193-208. [ Links ]
13. Cacho, J. L., García-García, R., Arcaya, J., Gay, F. J., Guerrero, A. L., & Gómez, J. C. (1996). El test del reloj en ancianos sanos. Revista de Neurología, 24, 1525-1528. [ Links ]
14. Chase, T. N., Burrows, G. H., & Mohr, E. (1987). Cortical glucose utilization patterns in primary degenerative dementias of the anterior and posterior type. Archives of Gerontology and Geriatrics, 6, 289-292. [ Links ]
15. Chen, S. T., Sultzer, D. L., Hinkin, C. H., Mahler, M. E. & Cummings, J. L. (1998). Executive dysfunction in Alzheimer's disease: Association with neuropsychiatric symptoms and functional impairment. Journal of Neuropsychiatry and Clinical Neurosciences, 10, 426-43. [ Links ]
16. Contador, I., Fernández-Calvo, B., Cacho, J., Ramos, F., & Hernández-Martín, L. (2009). La depresión en la demencia tipo Alzheimer: ¿Existe algún efecto sobre la cognición?. Revista de neurología, 49(10), 505-510. [ Links ]
17. Fink, G. R., Marshall, J. C., Halligan, P. W., Frith, C. D., Driver, J., Frackowiak, R. S., & Dolan, R. J. (1999). The neural consequences of conflict between intention and the senses. Brain, 122, 497-512. [ Links ]
18. Folstein, M. F., Folstein, S. E., & McHugh, P. R. (1975). Mini-Mental State: A practical method for grading the state of patients for the clinician. Journal of Psychiatric Research, 12, 189-198. [ Links ]
19. Galton, C. J., Patterson, K., Xuereb, J. H., & Hodges, J. R. (2000). Atypical and typical presentations of Alzheimer's disease: A clinical, neuropsychological, neuroimaging and pathological study of 13 cases. Brain, 123, 484-498. [ Links ]
20. Grady, C. L, Haxby, J. V, Horwitz, B., Sundaram, M., Berg, G., Schapiro, M.B., Rapoport, S.I. (1988). Longitudinal study of the early neuropsychological and cerebral metabolic changes in dementia of the Alzheimer type. Journal of Clinical and Experimental Neuropsychology, 10, 576-596. [ Links ]
21. Grady, C. L., Haxby, J. V., Schapiro, M. B., Gonzalez-Aviles, A., Kumar, A., Ball, M. J., Rapoport, S. I. (1990). Subgroups in dementia of the Alzheimer type identified using positron emission tomography. Journal of Neuropsychiatry and Clinical Neuroscience, 2, 373-384. [ Links ]
22. Habek, M., Hajnsek, S., Zarkovic, K., Chudy, D., & Mubrin, Z. (2010). Frontal variant of Alzheimer's disease: clinico-CSF-pathological correlation. Canadian Journal of Neurological Sciences, 37(1), 118-120. [ Links ]
23. Hachinski, V. C., Iliff, L. D., Zinhla, E., DuBoulay, G. H., McAllister, V., Marshall, L., Symon, L. (1975). Cerebral Blood flow in dementia. Archives of Neurology, 32, 632-637. [ Links ]
24. Holman, B.L., Johnson, K.A., Gerada, B., Carvalho, P.A., & Satlin, A., (1992). The scintigraphic appearance of Alzheimer' s disease: A prospective study using Technetium-99m-HMPAO SPECT. Journal of Nuclear Medicine 33 (2), 181-185. [ Links ]
25. Hughes, C. P., Berg, L., Danziger, W. L., Cohen, L. A., & Martin, R. L. (1982). A new clinical scale for staging of dementia. British Journal of Psychiatry, 140, 566-572. [ Links ]
26. Hutchinson, A. & Mathias, J. L. (2007). Neuropsychological deficits in frontotemporal dementia and Alzheimer' s disease: A meta-analytic review. Journal of Neurology, Neurosurgery, and Psychiatry, 78, 917-928. [ Links ]
27. Izal, M., & Montorio, I. (1994). Evaluación del medio y del cuidador del demente. En T. Del Ser y J. Peña Casanova (eds.). Evaluación neuropsicológica y funcional de la demencia, (pp. 201-212). Barcelona: Prous Science. [ Links ]
28. Jeong, Y., Han, D. H., Yi, H. A., Cho, S. S., Chin, J., Kang, S. J., Na, D. L. (2003). Neuropsychological and Neuroimaging Findings of Frontal Variant of Alzheimer's Disease. Journal of the Korean Neurological Association, 21 (1), 32-40. [ Links ]
29. Johnson, J. K., Head, E., Kim, R., Starr, A., & Cotman, C. W. (1999). Clinical and pathological evidence for a frontal variant of Alzheimer' s disease. Archives of Neurology, 56, 1233-1239. [ Links ]
30. Kanne, S. M., Balota, D. A., Storandt, M., McKeel, Jr., D. W., & Morris, J. C. (1998). Relating anatomy to function in Alzheimer's disease: neuropsychological profiles predict regional neuropathology 5 years later. Neurology, 50, 979-985. [ Links ]
31. Lafleche, G., & Albert, M. S. (1995). Executive function deficits in mild Alzheimer's disease. Neuropsychology, 9, 313-320. [ Links ]
32. Lambon, M. A., Patterson, K., Graham, N., Dawson, K., & Hodges, J. R. (2003). Homogeneity and heterogeneity in mild cognitive impairment and Alzheimer's disease: a cross-sectional and longitudinal study of 55 cases. Brain, 126, 2350-2362. [ Links ]
33. Larner, A. J. (2006). Frontal variant Alzheimer' s disease: A reappraisal. Clinical Neurology and Neurosurgery, 108(7), 705-708. [ Links ]
34. Mann, U. M., Mohr, E., Gearing, M., & Chase, T. (1992). Heterogeneity in Alzheimer' s disease: Progression rate segregated by distinct neuropsychological and cerebral metabolic profiles. Journal of Neurology, Neurosurgery, and Psychiatry, 55, 956-959. [ Links ]
35. McKhann, G., Drachman, D., Folstein, M., Katzman, R., Price, D., & Stadlan, E.M. (1984). Clinical diagnosis of Alzheimer's disease: Report of the NINCDS-ADRDA work group under the auspices of the Department of Health and Human Services task force on Alzheimer' s disease. Neurology, 34, 939-944. [ Links ]
36. Mega, M. S., Lee L., Dinov, I. D., Mishkin, F., Toga, A. W., & Cummings, J. L. (2000). Cerebral correlates of psychotic symptoms in Alzheimer's disease. Journal of Neurology, Neurosurgery, and Psychiatry. 69(2), 167-171. [ Links ]
37. Mohamed, S., Rosenheck, R., Lyketsos, C.G., & Schneider, L.S. (2010). Caregiver burden in Alzheimer disease: Cross-sectional and longitudinal patient correlates. American Journal of Geriatric Psychiatry, 18, 917-927. [ Links ]
38. Palmer, K., Lupo, F., Perri, R., Salamone, G., Fadda, L., Caltagirone, C., Cravello, L. (2011). Predicting disease progression in Alzheimer's disease: the role of neuropsychiatric syndromes on functional and cognitive decline. Journal of Alzheimer's Disease, 24(1), 35-345. [ Links ]
39. Perani, D., Di Piero, V., Vallar, G., Cappa, S., Messa, C., Bottini, G., Fazio, F. (1998). Technetium-99m HM-PAO-SPECT study of regional cerebral perfusion in early Alzheimer's disease. Journal of Nuclear Medicine, 29, 1507-1514. [ Links ]
40. Reed, B. R., Jagust, W. J., & Coulter, L. (1993). Anosognosia in Alzheimer' s disease: Relationships to depression, cognitive function, and cerebral perfusion. Journal of Clinical Experimental Neuropsychology, 15(2), 231-244. [ Links ]
41. Rey A (1987). Test de copia de la figura compleja. Madrid: TEA. [ Links ]
42. Ricci, M., Guidoni, S. V., Sepe-Monti, M., Bomboi, G., Antonini, G., Blundo, C., & Giubilei, F. (2009). Clinical findings, functional abilities and caregiver distress in the early stage of dementia with Lewy bodies (DLB) and Alzheimer's disease (AD). Archives of Gerontology and Geriatrics, 49(2), e101-e104. [ Links ]
43. Rinaldi, M. C., Piras, F., & Pizzamiglio, L. (2010). Lack of awareness for spatial and verbal constructive apraxia. Neuropsychologia, 48(6), 1574-1582. [ Links ]
44. Snowden, J. S., Stopford, C. L., Julien, C. L., Thompson, J. C., Davidson, Y., Gibbons, L., Mann, D. M. A. (2007). Cognitive phenotypes in Alzheimer's disease and genetic risk. Cortex, 43(7), 835-845. [ Links ]
45. Starkstein, S.E., Brockman, S., Bruce, D., & Petracca, G. (2010). Anosognosia Is a Significant Predictor of Apathy in Alzheimer' s disease. The Journal of Neuropsychiatry and Clinical Neurosciences, 378-383. [ Links ]
46. Starkstein, S. E., Jorge, R., Mizrahi, R., Adrian, J., & Robinson, R. G. (2007). Insight and danger in Alzheimer's disease. European journal of neurology, 14(4), 455-460. [ Links ]
47. Stopford, C. L., Snowden, J. S., Thompson, J. C., & Neary, D. (2008). Variability in cognitive presentation of Alzheimer's disease. Cortex, 44(2), 185-195. [ Links ]
48. Strauss, E., Sherman, E. M. S., & Spreen, O. (2006). A Compendium of neuropsychological tests: Administration, norms, and commentary. (3rd. ed.). NY. Oxford University Press. [ Links ]
49. Strub, R. L & Black, F. W. (2000). The Mental Status Examination in Neurology. F.A. Davis Company. [ Links ]
50. Swanberg, M. M., Tractenberg, R. E., Mohs, R., Thal, L. J. & Cummings, J. L., (2004). Executive dysfunction in Alzheimer disease. Archives of Neurology, 61, 556-560. [ Links ]
51. Taylor, K. I., Probst, A., Miserez, A. R., Monsch, A. U., & Tolnay, M. (2008). Clinical course of neuropathologically confirmed frontal-variant Alzheimer's disease. Nature Clinical Practice Neurology 4, 226-232. [ Links ]
52. Vilalta-Franch, J., Lozano-Gallego, M., Hernández-Ferrándiz, M., Llinás-Reglá, J., López-Pousa, S., & López, O.L. (1999). El inventario neuropsiquiátrico: propiedades psicométricas de su adaptación al castellano. Revista de Neurología, 29, 15-19. [ Links ]
53. Wallin, A., & Blennow, K. (1996). Clinical subgroups of the Alzheimer syndrome. Acta neurologica Scandinavica Supplementum, 165, 51-57. [ Links ]
54. Perry, R. J., Watson, P., & Hodges, J. R. (2000). The nature and staging of attention dysfunction in early (minimal and mild) Alzheimer's disease: Relationship to episodic and semantic memory impairment. Neuropsychologia 38, 252-271. [ Links ]
55. Wechsler, D. (1981). Wechsler Adult Intelligence Scale Revised. The Psychological Corporation, New York. [ Links ]
56. Wolk, D. A., Dickerson, B. C., Weiner, M., Aiello, M., Aisen, P., Albert, M. S., Wilks, K L. (2010). Apolipoprotein E (aPOE) genotype has dissociable effects on memory and attentional-executive network function in Alzheimer disease. Proceedings of the National Academy of Sciences of the United States of America, 107(22), 10256-10261. [ Links ]
57. Woodward, M., Brodaty, H., Boundy, K., Ames, D., Blanch, G., Balshaw, R., and PRIME Study Group (2010a). Does executive impairment define a frontal variant of Alzheimer's disease?. International Psychogeriatrics, 22, 1280-1290. [ Links ]
58. Woodward, M., Jacova, C., Black, S. E., Kertesz, A., Mackenzie, I. R., & Feldman, H. (2010b). Differentiating the frontal variant of Alzheimer' s disease. International Journal of Geriatric Psychiatry, 25(7), 732-738. [ Links ]
![]() Correspondence:
Correspondence:
Dr. Bernardino Fernández Calvo.
Departamento de Psicologia.
Universidade Federal da Paraíba,
Cidade Umversitána. Castelo Branco.
Joáo Pessoa, 58059-900, PB Brasil.
E-mail: bfcalvo@usal.es
Article received: 17-10-2011
reviewed: 10-1-2012
accepted: 18-1-2012