Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO  Similares en Google
Similares en Google
Compartir
Medicina Oral, Patología Oral y Cirugía Bucal (Ed. impresa)
versión impresa ISSN 1698-4447
Med. oral patol. oral cir. bucal (Ed.impr.) vol.9 no.5 nov./dic. 2004
Consensus statement on antimicrobial treatment of odontogenic bacterial infections
BASCONES A, AGUIRRE JM, BERMEJO A, BLANCO A, GAY-ESCODA C, GÓNZÁLEZ-MOLES MA ET AL. CONSENSUS STATEMENT ON ANTIMICROBIAL TREATMENT OF ODONTOGENIC BACTERIAL INFECTIONS. MED ORAL PATOL ORAL CIR BUCAL 2004;9:363-76.
SUMMARY
The infection of the oral cavity is a common public health problem and constant cause for antibiotic prescription, with 10% of antibiotics used to treat this problem. However, few studies have so far aimed to determine its incidence. Added to this, its relationship with certain sytemic diseases (cardiac, endocrine, etc…) confers this pathology vital importance. In spite of the frequency and importance of odontogenic infection, the current dispersion in criteria regarding key aspects in classification, terminology and therapeutic recommendations is noticeable. The main objective of this document, compiled as a consensus statement by specialists in microbiology and odontology, is to establish useful recommendations for all of those involved in the clinical management of this pathology. Special attention has been placed on the rise in bacterial resistance observed over the last years, specifically the proliferation of betalactamase producing strains. Another important factor causing the resistance to appear is lack of therapeutic compliance, specially what regards dosage and treatment duration. Therefore, this pathology constitutes a complex problem which requires the instauration of broad spectrum antimicrobials, well tolerated and a convenient posology so that patients receive the adequate dose over the necessary period. High doses of amoxicillin/clavulanate (2000 mg / 125 mg) have showed good results and power to overcome resistance. Other agents such as metronidazole and clindamycin, followed by de claritromycin and azithromycin have also proved to be active against most of microorganisms responsible for odontogenic infection.
Key words: Odontogenic infections, classification, diagnose, treatment, odontogenic microorganisms, resistance, antimicrobial agents, antibiotics, amoxicilin, clawlanic acid.
1. INTRODUCTION
Although there is very little data regarding the incidence of infections of the oral cavity, no one doubts their relevance. Of these types of infections, odontogenic infections (infections that involve tooth and periodontal tissues) are the most common. It is the most frequent reason for seeking odontological consultation and intervention and it affects the entire population from childhood (especially cavities) throughout a person's entire lifespan (periodontitis, implant complications, etc.), which entails a considerable impact both on public health in general, as well as the economic resources destined to maintain public health. It has been estimated that odontogenic infections in Spain represent approximately 10% of all antibiotic prescriptions(1,2)
Scientific evidence has revealed a relationship between some serious oral infections and specific systemic cardiovascular(3), lung and endocrine (diabetes mellitus) diseases, as well as with alterations during pregnancy(4,5). Because of this association between infection and other systemic diseases, it is essential that odontogenic infections be avoided as much as possible or failing that, that they be identified and treated promptly and appropriately. On occasion, an odontogenic infection can spread and provoke polymicrobial infections in other locations, such as the paranasal sinuses (odontogenic maxillary sinusitis), cervicofacial subaponeurotic spaces, palate, central nervous system (cerebral abscess), endocardium (endocarditis), etc.(6)
However, and despite the frequency and importance of odontogenic infections, when undertaking a review of the literature, the dispersion of criteria in key aspects such as terminology, classification, treatment recommendations, etc. is surprising, as is the paucity of papers in prestigious publications, making it impossible to establish an appropriate level of scientific evidence. Hence, this document is the result of bibliographic review, but, above all, it represents the fruit of the experience accumulated over many years of the participating specialists and of the group discussions held for the purpose of drafting it.
The main objective of this document, which has been elaborated by specialists representing 10 public universities in Spain in collaboration with specialists in the microbiology of these kinds of infections, is none other than to establish recommendations that will be of use for all those involved in the daily clinical management of patients suffering from these diseases.
2. CLASSIFICATION OF ODONTOGENIC INFECTIONS OF THE ORAL CAVITY
Infections of mixed aetiology affecting the oral cavity can be classified into two main groups on the basis of origin: a) Odontogenic: cavities, pulpitis, periapical abscess, gingivitis, periodontitis, pericoronitis, osteitis, and infection of the subaponeurotic spaces; and b) Non-odontogenic: infections of the oral mucosa, infections of the salivary glands, etc (7).
In 1999, the American Academy of Periodontology organized an international task force to create a classification of periodontal diseases and conditions (8) in response to the criticisms of previous classifications (9) (obscure diagnostic criteria, overlapping of disease-related groups, too much importance given to the patient's age, onset of illness and rate of progression, which are often difficult to determine).
The odontogenic infections that present most frequently would be those that result from dental cavities, dentoalveolar infections (infections of the pulpa and periapical abscesses), gingivitis (including necrotising ulcerative gingivitis), periodontitis (including pericoronitis and the periimplantitis), infections of the sub-aponeurotic spaces, osteitis, and osteomyelitis.
3. WHAT ARE THE MOST IMPORTANT MICROORGANISMS IN ODONTOGENIC INFECTION?
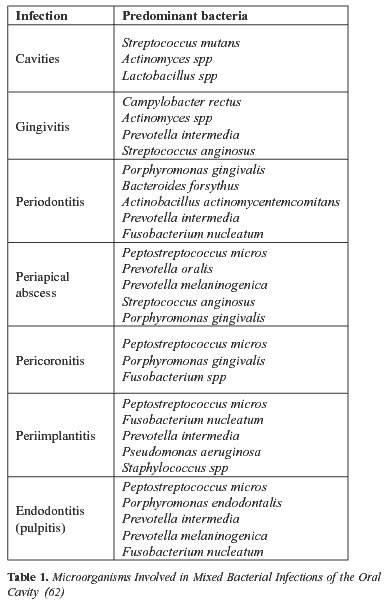
The oral cavity is a complex ecosystem made up of more than 500 bacterial species (10). Overall, the Streptococcus, Peptostreptococcus, Veillonella, Lactobacillus, Corynebacterium and Actinomyces genera represent more than 80% of all cultivable flora (11). In the aetiology of periodontal disease, a whole series of species such as Actinobacillus actinomycetemcomitans, Porphyromonas gingivalis, Prevotella intermedia and Tannerella forsythensis can be especially highlighted due to their frequency and the importance of the complications that may arise from them. Facultative gram-negative bacilli are uncommon in healthy adults and are seen almost exclusively in elderly, hospitalised patients with serious medical diseases (12).
The ploymicrobial nature of odontogenic infection has been demonstrated in many papers. For example, in a study conducted by Brook et al.(13) in 32 patients with periapical abscess, 78 bacterial isolates were obtained (55 anaerobic and 23 aerobic), with a mean of 2.4 isolates per sample. Only anaerobic bacteria were found to be present in 16 patients (50%), only aerobic in 2 (6%) and mixed aerobic and anaerobic flora, in 14 (44%). The main isolates consisted of bacteria belonging to the Peptostreptococcus, Prevotella and Porphyromonas genera. Of the facultative anaerobic bacteria, oral streptococci are the most frequent.
Table 1 shows the most commonly found bacteria in each oral condition.
4. WHEN IS COMPLEMENTARY DIAGNOSTIC TESTING INDICATED?
The diagnosis of odontogenic infection is based on anamnesis, observation and examination that allows symptoms and signs to be recorded. Information regarding the patient's history of the following conditions is essential, as it will necessarily influence treatment and prophylaxis: endocarditis, implants, diabetes, immunodepression, etc. Radiological diagnosis is fundamental in determining the location, extension and possible complications of these lesions.
The role of the laboratory in diagnosing odontogenic infections in routine practice in dentists' offices is controversial. Non-specific analytical data (leucocytes, complement, lymphocytes, immunoglobulins, glycaemia, etc.) must be requested when dealing with repeated or unusual infections or infections that are suspicious of any underlying disease that can have repercussions in the oral cavity. Better still, the internist's report should be requested before undertaking any kind of action. The patient can be spared serious medical complications and the professional can avoid legal complications. Bear in mind conditions such as endocarditis, diabetes, AIDS, hepatitis, etc.
Insofar as microbiological studies are concerned, pathology samples will be taken prior to commencing with antibiotic treatment and will be sent to the laboratory following proper standards. The rapid techniques currently on the market can be a great diagnostic aid. The microbiological diagnosis seeks to rule out a specific aetiology, identify the aetiology of the condition and obtain overall information that is currently lacking, as well as to determine sensitivity to antimicrobial agents. These data will be useful in deciding on the treatment to be administered, whether to effect a change in the event that the empirical treatment fails and to establish general empirical therapies.
5. THERAPEUTIC MANAGEMENT OF ODONTOGENIC INFECTION
The issue of odontogenic infection must be approached from three, mutually complementary treatment areas. Aetiological odontological treatment, which often includes surgical interventions of varying magnitude and requiring different levels of professional expertise; systemic support treatment, which covers a broad spectrum ranging from symptomatic pain management and controlling the inflammation, all the way to physical measures, hydration, fever control, glycaemic control, etc. Finally, antimicrobial treatment should only be applied on rare occasions and on the basis of rational, efficiency criteria. In general, antimicrobial treatment must be initiated whenever the condition presents clear clinical manifestations of infection. Antimicrobial treatment of odontogenic infections aims to prevent local spread and spread to neighbouring areas, to decrease the bacterial inoculum in the infectious focus and to prevent complications derived from disemination via the circulatory system (14,15). Antimicrobial treatment is not the only treatment option for odontogenic infection, since antibiotic administration alone is often not sufficient to eradicate the infection. Depending on the infection and the patient's characteristics, the optimum treatment for a give infection may require systemic or local antimicrobial agents, odontological treatment or surgery, or a combination of the above (6,16,17) .
6. IN WHAT SITUATIONS IS ANTIMICROBIAL TREATMENT WARRANTED?
Not all odontogenic infections require antimicrobial treatment. In some cases, surgical treatment is also necessary and in others, the best course of treatment is debridement, irrigation and drainage.
-Endodontic Infections Arising from the Pulpa
In some situations, acute endodontic treatment can be complemented with systemic antibiotics, as well as with analgesics and/ or anti-inflammatory drugs (18). Antibiotics are also indicated in cases in which the patient is immunodepressed and requires prophylaxis.
-Chronic Gingivitis and Necrotising Ulcerative Gingivitis (NUG)
Generally speaking, the treatment of mild gingivitis does not include systemic antibiotic administration. It requires local treatment to eliminate dental plaque and to disinfect the gingival grooves. Useful measures include rinsing with chlorhexidine, brushing with a mixture of sodium bicarbonate and hydrogen peroxide, and/ or frequent rinsing with saltwater.
One exception is NUG, in which systemic antibiotic use is recommended. The same is true of streptococcal gingivitis, caused by group A beta haemolytic streptococcus (Streptococcus pyogenes) that presents as a complication of acute streptococcal pharyngitis/ tonsillitis, in which active antibiotics should be used against this microorganism (19). In the case of NUG, in addition to antibiotic treatment, debridement with ample irrigation is recommended (16). Topical application of mouthwash containing chlorhexidine or saline solution is effective in controlling the pain and ulceration that accompanies this condition.
- Periapical Abscess
This comprises a clear indication for debridement and surgical drainage complemented with systemic antibiotics.
-Periodontal Abscess
Treatment consists of debriding and draining the purulent pocket. Antibiotic treatment is reserved for those situations with there is local or systemic dissemination.
-Periodontitis
Debridement, elimination of the calculus and root planning to remove subgingival plaque deposits constitute the first line of treatment. Subgingival irrigation should also be performed using ultrasound tartar removal equipment to disinfect the gingival sulcus. Other useful measures consist of rinsing the mouth with chlorhexidine or brushing with a mixture of sodium bicarbonate and hydrogen peroxide. Systemic antibiotics are indicated especially for the treatment of aggressive periodontitis (16).
-Pericoronitis
Systemic antibiotics are almost always necessary to keep the infection from spreading. Local treatment consisting of debridement, irrigation and drainage of the affected areas, or even tooth extraction can also be performed.
-Periimplantitis
Systemic antibiotic therapy in certain cases may be accompanied by mechanical debridement. Rinsing the mouth with chlorhexidine for 30 seconds after brushing teeth may also be useful as coadjuvant treatment (20).
-Severe Infections of the Fascia and Deep Head and Neck Tissues
The treatment of infections located in the cervicofacial aponeurotic spaces include the following measures: 1) aetiological treatment, 2) incision, debridement and drainage of purulent accumulations and 3) antibiotic therapy. Odontogenic infections are caused by a highly predictable group of bacteria, so the first choice of antibiotic is made empirically. However, if evolution is unfavourable, the antibiotic chosen can be substituted by another one or more than one after identifying the causal microorganisms by means of culture and antibiogram typing. 4) Finally, complementary systemic care is also required (hydration, nutritional support, analgesics, antipyretics and anti-inflammatory drugs).
Attention must be paid at all times to alert criteria that indicate the need to transfer the patient to a hospital, possibly on an emergency basis (Table 2).
7. WHAT CHARACTERISTICS MUST THE IDEAL ANTIBIOTIC HAVE FOR THE TREATMENT OF ODONTOGENIC INFECTION?
The ideal antibiotic for treating an infection must have a series of characteristics such as: a) it must be active against the microorganisms involved in the infection; b) it must meet appropriate pharmacokinetic parameters (good penetration and diffusion at the site of infection); c) it must be well tolerated and have few adverse effects (21), and d) it must allow for a dosing schedule that facilitates treatment compliance.
The ploymicrobial component of odontogenic infection advises the use of antibiotics that are active against both aerobic and anaerobic bacteria are recommended, requires that the proper antibiotic be used for treatment. It is often necessary to administer combinations of antibiotics that can achieve a spectrum of activity and are more appropriate to the type of infection.
8. HOW SENSITIVE ARE THE PATHOGENS INVOLVED IN ODONTOGENIC INFECTION TO THE MOST COMMONLY USED ANTIMICROBIAL AGENTS?
The increased prevalence of bacterial resistance means that antibiotics that have been useful in the past are currently no longer as effective as they once were, as is the case with certain dose levels. In this regard, in the last 10-15 years the number of resistant microorganisms in the oral cavity has doubled (22). We can cite the following example: studies have revealed the presence of beta-lactamase producing species in 74-88% of patients with periodontitis (23,24). Likewise, over the course of recent years as seen with other pathogens such as Streptococcus pneumoniae, the levels of resistance to macrolides, beta-lactamase and clindamycin of several viridans group streptococci species have increased notably (25-28) . Whereas an increase in the macrolide doses does not lead to increased coverage against the resistant strains, in the case of beta-lactam, higher doses can lead to better coverage (29).
Table 3 shows the activity of several antimicrobial agents against the most important microorganisms that cause periodontal disease.
9. WHICH ANTIBIOTICS AND WHAT DOSES ARE ADEQUATE FOR TREATING ODONTOGENIC INFECTION?
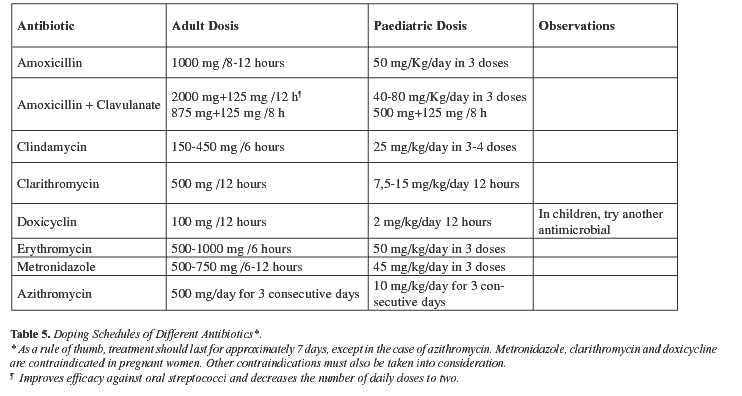
Treatment duration with antibiotics depends on the type of infection, the extension of the condition and on the antibiotic chosen. Overall, treatment duration will vary between 5 and 10 days; in other words, treatment should continue for 3 or 4 days after clinical manifestations have disappeared (30).
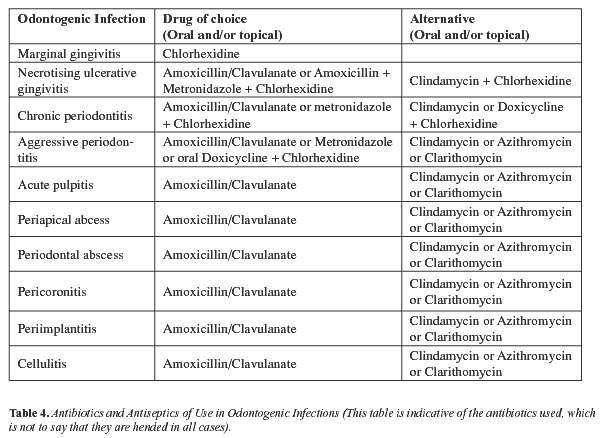
Amoxicillin, amoxicillin/ clavulanate, cephalosporins, doxicycline, metronidazole, clindamycin and macrolides, such as erythromycin, clarithromycin and azithromycin, all stand out amongst the large variety of systemic antimicrobials used to treat odontogenic infection. Tables 4 and 5 present antimicrobial agents and the dosing schedules recommended for each indication.
Penicillins
Penicillin, ampicillin and amoxicillin are bactericides that are useful in treating the acute phase of odontogenic infection, in addition to preventing associated complications (7). Due to their effectiveness against facultative aerobic and anaerobic pathogens, they are considered to be the antibiotics of choice in the treatment of infections of mixed aetiology in the oral cavity (31). However, there are more and more beta-lactamase producing bacteria, enzymes that are capable of hydrolysing penicillins and, therefore, leading to treatment failure (32-34) particularly when strains of the Prevotella, Porphyromonas and Fusobacterium genera are present (35-37).
In fact, penicillin administration has been linked to the appearance of beta-lactamase producing bacilli in the oropharynx (38,39).Amoxicillin and ampicillin increase penicillin's spectrum to cover enteric gram-negative bacilli. Amoxicillin is better than ampicillin because of its superior enteric absorption (60-80% versus 30-55%) (40, 41).
Given the increased prevalence of beta-lactamase producing microorganisms, the association of a penicillin with a beta-lactamase inhibitor such as amoxicillin/ clavulanic acid has become the treatment of choice in many of these conditions (42,43). The increased resistance of some species of oral streptococci indicates that high doses of amoxicillin be used to treat infections in which these pathogens might be involved. In this regard, a new pharmacokinetically enhanced formulation of amoxicillin/ clavulanate has been developed (amoxicillin/ clavulanate, 1000/62.5 mg) that, in addition to lowering the number of daily doses to two, also eradicates strains considered to be resistant to conventional formulations (44-46). Furthermore, this new formulation, when administered along with high doses of Amoxicillin, can delay or decrease the risk of increasing the prevalence rate of oral streptococci resistance, as seen in children with Streptococcus pneumoniae and a high dosis, short course of treatment with amoxicillin (5-7 days) (47,48).
Cephalosporins
Cephalosporins are classified in generations, based on their antibacterial spectra, regardless of when they were synthesised. In general, as we move further along the generations, activity against gram-negative germs improves, while effectiveness against gram-positive germs decreases (49). They present the disadvantage of having very poor activity against anaerobic bacteria, with the exception of cephamycins (cefoxitine, cefminox and cefotetan) for which there are no oral formulations (21).
Tetracyclines
Tetracyclines have classically been the standard antibiotic of use in treating odontogenic infection, although at present, they exert limited activity as a result of increased resistance, particularly in countries such as Spain where there is a high level of antimicrobial use (50). Because of their high affinity for bone and dental tissue, its use is not recommended during pregnancy, while nursing or in children less than eight years of age, since when deposited on teeth and bones during development they can produce alterations such as dental hypoplasia, bone deformities and abnormal tooth colour (51).
Nitroimidazoles
Metronidazole, ornidazole and tinidazole are antibiotics with excellent activity against anaerobic gram-negative bacilli and spirochete, but hardly act, if they act at all, against anaerobic cocci and facultative, aerobic bacteria of the oral cavity (7,52). They should be administered in combination with other antibiotics in mixed infections of the oral cavity that involve oral aerobic or facultative streptococci.
Lincosamides
Clindamycin continues to be the treatment of choice in patients who are allergic to beta-lactams in most odontogenic infections. It presents a good level of activity against anaerobic bacteria, although more and more resistant strains are emerging (53,54). More than 25% of the viridans group streptococci present a high degree of resistance (55) that cannot be overcome with high doses of this antibiotic, nor is it active against some gram-negative bacilli, such as A. actinomycetemcomitans, Eikenella corrodens and Capnocytophaga spp (56-58).
Macrolides
Macrolides are bacteriostatic antibiotics with a spectrum of activity that covers gram-positive bacteria, some gram-negative bacilli, bacteria growing intercellularly and several anaerobic bacteria, including Porphyromonas and Prevotellagenera. Bacteroides spp and Fusobacterium spp tend to be resistant to these antibiotics (59). Like other streptococci species (S. pneumoniae, Streptococcus pyogenes) (60), the prevalence of resistance to oral streptococci has increased significantly, with rates of more than 50% in many areas of our country (55,61).
Amongst representatives of this drug family, clarithromycin show the greatest in vitro activity against anaerobic gram-positive bacilli and azithromycin, against anaerobic gram-negative bacilli.
CONCLUSIONS
1. There are a host of microorganisms in the oral cavity whose taxonomy is difficult to ascertain and it is not always easy to determine how they relate to clinical presentations.
2. Microbial- and host-related factors play a role in oral and facial infections, which means that the response obtained in vivo may differ from what occurs in vitro.
3. Many oral bacteria produce beta-lactamases, which can sometimes complicate antibiotic therapy.
4. There are some individuals who are especially susceptible and in whom microorganisms produce more severe clinical symptoms and are more resistant to certain treatments.
5. Certain factors alter patients' susceptibility to different microorganisms (age, blood dyscrasias, drug treatment, hospitalisation, avitaminosis and others).
6. Antibiotic efficacy is multifactorial and success depends on different parameters being met, such as dosing schedule, time, etc.
7. Amoxicillin/ clavulanate, metronidazole and clindamycin are active against most of the microorganisms that are responsible for odontogenic infections. Other alternatives, such as clarithromycin and azithromycin, complete the therapeutic arsenal.
REFERENCES
1. Machuca M, Espejo J, Gutiérrez L, Herrera J. Análisis de la prescripción antibiótica en una farmacia comunitaria. Pharm Care Esp 2000;2:411-9. [ Links ]
2. Intercontinental Marketing Services Ibérica, S.A. 2003; Madrid. España. [ Links ]
3. Beck JD, Pankow J, Tyroler HA, Offenbacher S. Dental infections and atherosclerosis. Am Heart J 1999;138:528-33. [ Links ]
4. Offenbacher S, Beck J. Periodontitis: A potential risk factor for spontaneous preterm birth. Compend Contin Educ Dent 1998;19:32-9. [ Links ]
5. Loesche WJ. Association of the oral flora with important medical diseases. Curr Opin Periodontol 1997;4:21-8. [ Links ]
6. Gay-Escoda C, Berini Aytés L, eds. Tratado de Cirugía Bucal. Tomo I. Madrid: Ergon; 2004. [ Links ]
7. Maestre JR. Infecciones bacterianas mixtas de la cavidad oral. Enferm Infecc Microbiol Clin 2002;20:98-101. [ Links ]
8. Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol 1999;4:1-6. [ Links ]
9. Caton JF. Periodontal diagnosis and diagnostic aids. En: World workshop in clinical periodontics. Princeton, NJ: American Academy of Periodontology; 1989. p. 1-22. [ Links ]
10. Valle Rodríguez JL, Gómez-LusCentelles ML, Prieto Prieto J, Liébana Ureña J. Composición y ecología de la microbiota oral. En: Liébana Ureña J. eds. Microbiología oral. Madrid: Interamericana McGraw-Hill; 1995. p. 402-7. [ Links ]
11. Chow AW. Infections of the oral cavity, neck, and head. En: Mandell GL, Bennett JE, Dolin R, eds. Principles and Practice of infectious diseases. 5th edition. Philadelphia: Churchill Livingstone; 2000. p. 689-701. [ Links ]
12. Valenti WM, Trudell RG , Bentley DW. Factors predisposing to oropharyngeal colonization with gramnegative bacilli in the aged. N Engl J Med 1978;298:1108. [ Links ]
13. Brook I, Frazier EH, Gher ME. Aerobic and anaerobic microbiology of periapical abscess. Oral Microbiol Immunol 1991;6:123-5. [ Links ]
14. Cianco SG. Antiseptics and antibiotics as chemotherapeutic agents for periodontitis management. Compend Contin Educ Dent 2000;21:59-62. [ Links ]
15. Slots J, Jorgensen MG. Efficient antimicrobial treatment in periodontal maintenance care. J Am Dent Assoc 2000;131:1293-304. [ Links ]
16. Loesche WJ, Grossman NS. Periodontal disease as a specific, albeit chronic, infection: diagnosis and treatment. Clin Microbiol Rev 2001;14:727-52. [ Links ]
17. Jorgensen MG, Slots J. Practical antimicrobial periodontal therapy. Compend Contin Educ Dent 2000;21:111-6. [ Links ]
18. Siqueira JF. Endodontic infections: Concepts, paradigms, and perspectives. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2002;94:281-93. [ Links ]
19. Ciancio SG, Van Winkelhoff AJ. Antibiotics in periodontal therapy. En: Newman MG, Winkelhoff AJ, eds. Antibiotics and antimicrobial use in dental practice. Illinois: Quintessence Publishing Co, Inc; 2001. [ Links ]
20. Quinteros M, Delgado E, Sánchez MA, Berini L, Gay-Escoda C. Estudio microbiológico de la periimplantitis. Presentación de 9 casos clínicos. Av Periodoncia Implantol Oral 2000;12:137-50. [ Links ]
21. Liñares J, Martín-Herrero JE. Bases farmacomicrobiológicas del tratamiento antibiótico de las enfermedades periodontales y periimplantarias. Av Odontoestomatol 2003;(especial):23-33. [ Links ]
22. Walker CB. The acquisition of antibiotic resistance in the periodontal pathogens. Periodontol 2000 1996;10:79-88. [ Links ]
23. Herrera D, Van Winkelhoff AJ, Dellemijn-Kippuw N, Winkel EG, Sanz M. Beta-lactamase producing bacteria in the subgingival microflora of adult patients with periodontitis. A comparison between Spain and the Netherlands. J Clin Periodontol 2000;27:520-5. [ Links ]
24. Van Winkelhoff AJ, Winkel EG, Barendregt D, Dellemijn-Kippuw, Stijne A, Van der Velden U. Beta-lactamase producing bacteria in adult periodontitis. J Clin Periodontol 1997;538-43. [ Links ]
25. Alcaide F, Liñares J, Pallares R, Carratala J, Benítez MA, Gudiol F, et al. In vitro activities of 22 beta-lactam antibiotics against Penicillin-Resistant and Penicillin-susceptible viridans group streptococci isolated from blood. Antimicrob Agents Chemother 1995;39:2243-7. [ Links ]
26. Ioannidou S, Tassios PT, Kotsovili-Tseleni A, Foustoukou M, Legakis NJ, Vatapoulos A, et al. Antibiotic resistance rates and macrolide resistance phenotypes of viridans group streptococci from the oropharynx of healthy Greek children. Int J Antimicrob Agents 2001;17:195-201. [ Links ]
27. Doern GV, Ferraro MJ, Brueggemann AB, Ruoff KL. Emergence of high rates of antimicrobial resistance among viridans group streptococci in the United States. Antimicrob Agents Chemother 1996;40:891-4. [ Links ]
28. Aracil B, Miñanbres M, Oteo J, Torres C, Gómez-Garces JL, Alos JI. High prevalence of erythromycin-resistant and clindamycin-susceptible (M phenotype) viridans group streptococci from pharyngeal samples: a reservoir of mef genes in commensal bacteria. J Antimicrob Chemother 2002;48:587-95. [ Links ]
29. Prieto J, Martín-Herrero JE, García-Rey C. Relación entre consumo de antibióticos y selección de resistencia en el género Streptococcus. Medicina Preventiva 2002;8:23-30. [ Links ]
30. Bascones A, Manso FJ. Tratamiento de las infecciones orofaciales de origen bacteriano. En: Bascones A, Manso FJ, eds. Infecciones orofaciales. Diagnóstico y tratamiento. Madrid: Avances Médico-Dentales; 1994. p. 89-116. [ Links ]
31. Kuriyama T, Karasawa T, Nakagawa K, Saiki Y, Yamamoto E, Nakamura S. Bacteriologic features and antimicrobial susceptibiliry in isolates from orofacial odontogenic infections. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2000;90:600-8. [ Links ]
32. Legg JA, Wilson M. The prevalence of beta-lactamase producing bacteria in subgingival plaque and their sensitivity to Augmentin. Br J Oral Maxillofac Surg 1990;28:180-4 [ Links ]
33. Heimdahl A, Von Konow L, Nord CE. Beta-lactamase-producing Bacteroides species in the oral cavity in relation to penicillin therapy. J Antimicrob Chemother 1981;8:225-9. [ Links ]
34. Kinder SA, Holt SC, Korman KS. Penicillin resistance in subgingival microbiota associated with adult periodontitis. J Clin Microbiol 1986;23:1127-33. [ Links ]
35. Aldridge KE, Ashcraft D, Cambre K, Pierson CL, Jenkins SG, Rosemblatt JE. Multicenter survey of the changing in vitro antimicrobial susceptibilities of clinical isolates of Bacteroides fragilis group, Prevotella, Fusobacterium, Porphyromonas, and Peptostreptococcus species. Antimicrob Agents Chemother 2001;45:1238-43 [ Links ]
36. Fosse T, Madinier I, Hitzig C, Charbit Y. Prevalence of beta-lactamase-producing strains among 149 anaerobic gram-negative rods isolated from periodontal pockets. Oral Microbiol Immunol 1999;14:352-7. [ Links ]
37. Wexler HM, Molitoris E, Molitoris D. Susceptibility testing of anaerobes: old problems, new options?. Clin Infect Dis 1997;25:275-8. [ Links ]
38. Brook I, Gober AE. Emergence of beta-lactamase-producing aerobic and anaerobic bacteria in the oropharynx of children following penicillin chemotherapy. Clin Pediatr 1984;23:338-41. [ Links ]
39. Turner K, Nord CE. Emergence of beta-lactamase producing microorganisms in the tonsils during penicillin treatment. Eur J Clin Microbiol 1986;5:399-404. [ Links ]
40. Muñoz Bellido JL, Alonso MA, Gutiérrez MN. Penicilinas. En: García Sanchez JE, López R, Prieto J, eds. Antimicrobianos en Medicina. Barcelona:Prous Science; 1999. p. 41-71. [ Links ]
41. Marín M, Gudiol F. Antibióticos betalactámicos. Enferm Infecc Microbiol Clin 2003;21:42-5. [ Links ]
42. Abu-Fanas SH, Dricker DB, Hull PS. Amoxicillin with clavulanate acid and tetracycline in periodontal therapy. J Dent 1991;19:97-9. [ Links ]
43. Todd PA, Benfield P. Amoxicillin/clavulanic acid. An update of its antimicrobial activity, pharmacokinetic properties and therapeutic use. Drugs 1990;39:264-307. [ Links ]
44. Kaye CM, Allen A, Perry S, McDonagh M, Davy M, Storm K, et al. The clinical pharmacokinetics of a new pharmacokinetically enhanced formulation of amoxicillin/clavulanate. Clin Ther 2001;23:578-84. [ Links ]
45. Jacobs MR. How can we predict bacterial eradication?. Int J Infect Dis 2003;7:S13-20. [ Links ]
46. Martínez Lacasa J, Jiménez J, Ferrás V, García-Rey C, Bosom M, Sola-Morales O, et al. A Double Blind, Placebo-Controlled, Randomised, Comparative Phase III Clinical Trial of Pharmacokinetically Enhanced Amoxicillin/Clavulanate 2000/125, as Prophylaxis or as Treatment vs Placebo for Infectious and Inflammatory Morbidity after Third Mandibular Molar Removal (TMR). 43rd Annual ICAAC Chicago. September 2003. [ Links ]
47. Garau J, Twynholm M, García-Méndez E, Siquier B, Rivero A and the 557 Clinical Study Group. Oral pharmacokinetically enhanced co-amoxiclav 2000/125 mg, twice daily, compared with co-amoxiclav 875/125 mg three times daily, in the treatment of community-acquired pneumonia in European adults. J Antimicrob Chemother 2003;52:826-36. [ Links ]
48. Schrag SJ, Peña C, Fernández J. Effect of short-course, high-dose amoxicillin therapy on resistant pneumococcal carriage. A randomized trial. JAMA 2001;286:49-56. [ Links ]
49. Marín M, Gudiol F. Antibióticos betalactámicos. Enferm Infecc Microbiol Clin 2003;21:42-5 [ Links ]
50. Van Winkelhoff AJ, Herrera González D, Winkel EG, Dellemijn-Kippuw N, Vandenbroucke-Grauls CM, Sanz M. Antimicrobial resistance in the subgingival microflora in patients with adult periodontitis. A comparison between The Netherlands and Spain. J Clin Periodontol 2000;27:79-86. [ Links ]
51. Pérez Trallero E, Iglesias L. Tetraciclinas, sulfamidas y metronidazol. Enferm Infecc Microbiol Clin 2003;21:520-9. [ Links ]
52. Gómez García AC, Blanco Roca MT, Morán Domínguez FJ. Nitroimidazoles. En: García Sánchez JE, López R, Prieto J, eds. Antimicrobianos en Medicina. Barcelona. Prous Science; 1999. p. 377-82. [ Links ]
53. Koeth LM, Good CE, Appelbaum PC. Surveillance of susceptibility patterns in 1297 European and US anaerobic and capnophilic isolates to co-amoxiclav and five other antimicrobial agents. J Antimicrob Chemother 2004; Advance Access published May 5. [ Links ]
54. Turner PJ, Edward JR. A compilation of studies assessing the in vitro activity of meropenem and comparators in 84 laboratories throughout Europe. Clin Microbiol Infect 1997;3:32-50. [ Links ]
55. Rodríguez-Avial I, Rodríguez Avial C, Culebras E. Distribution of mef(A) and erm(B) genes in macrolide-resistant blood isolates of viridans group streptococci. J Antimicrob Chemother 2001;47:727-8 [ Links ]
56. Mensa J, Gatell JM, Jiménez de Anta MT, Prats G, Domínguez-Gil A,eds. Guía de terapéutica antimicrobiana 2002. Barcelona: Masson. [ Links ]
57. Gordon JM, Walker CB. Current status of systemic antibiotic usage in destructive periodontal disease. J Periodontol 1993;64:760-71 [ Links ]
58. Eick S, Pfister W, Fiedler D, Straube E. Clindamycin promotes phagocytosis and intracelluar killing of periodontopathogenic bacteria by crevicular granulocytes: an in vitro study. J Antimicrob Chemother 2000;46:583-8 [ Links ]
59. Mensa J, García-Vázquez E, Vila J. Macrólidos, cetólidos y estreptograminas. Enferm Infecc Microbiol Clin 2003;21:200-8. [ Links ]
60. Pérez-Trallero E, Fernández-Mazarrasa C, García-Rey C, Bouza E, Aguilar L, García-de-Lomas J, et al, Spanish Surveillance Group for Respiratory Pathogens. Antimicrobial susceptibilities of 1,684 Streptococcus pneumoniae and 2,039 Streptococcus pyogenes isolates and their ecological relationships: results of a 1-year (1998-1999) multicenter surveillance study in Spain. Antimicrob Agents Chemother 2001;45:3334-40. [ Links ]
61. Pérez-Trallero E, Vicente D, Montes M, Marimón JM, Piñeiro L. High proportion of pharyngeal carriers of comensal streptococci resistant to erythromycin in Spanish adults. J Antimicrob Chemother 2001;48:225-9. [ Links ]
62. Prieto J, Maestre JR. Tratamiento de las infecciones de etiología mixta. En: Bascones A, Perea EJ, eds. Infecciones orofaciales. Volumen 2. Madrid: Dentisnet; 2003. [ Links ]











 texto en
texto en