Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Pharmacy Practice (Granada)
versión On-line ISSN 1886-3655versión impresa ISSN 1885-642X
Pharmacy Pract (Granada) vol.14 no.3 Redondela jul./sep. 2016
https://dx.doi.org/10.18549/PharmPract.2016.03.785
ORIGINAL RESEARCH
A pharmacy-based medication reconciliation and review program in hemodialysis patients: a prospective study
Nicholas J. Patricia1 and Edward F. Foote2
1 Nicholas J. Patricia. Pharm.D. Department of Pharmacy Practice, Nesbitt School of Pharmacy, Wilkes University. Wilkes-Barre, PA (United States). nicholas.patricia@wilkes.edu
2 Edward F. Foote. Pharm.D. Department of Pharmacy Practice, Nesbitt School of Pharmacy, Wilkes University. Wilkes-Barre, PA (United States). edward.foote@wilkes.edu
ABSTRACT
Background: Hemodialysis (HD) patients are on multiple medications, see many prescribers and have many hospitalizations which put them at risk for medication record discrepancies and medication related problems (MRP). Being able to effectively identify and reconcile these medication issues is crucial in reducing hospitalizations, morbidities, and mortalities. The care of the hemodialysis patients can be enhanced by incorporating a pharmacist into the interprofessional team. There is little data in the literature on medication record discrepancies and MRP's in dialysis patients.
Objective: The objectives of this research were to determine the types of medication discrepancies and MRPs in dialysis patients and if recommendations for changes based on these findings were accepted by providers.
Methods: Patients were asked to bring medications to the dialysis unit for review. Discrepancy and MRP recommendations were communicated to the unit staff via written progress notes. A follow-up was performed an average of 33 days later to determine if the recommendations were accepted.
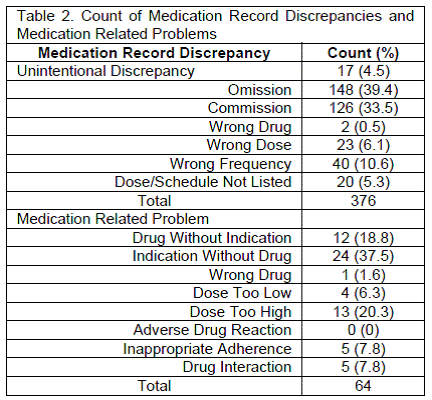
Results: Overall, in 93 unique patients, 376 discrepancies (3.1 per patient) and 64 MRPs (0.5 per patient) were identified. The most common type of discrepancy and MRP was drug omission and indication without drug, respectively. Of the total 440 interventions, 77% were ultimately accepted. Discrepancies were more likely to be accepted as compared to MRPs (85% vs. 27%, respectively).
Conclusion: Medication record discrepancies and MRPs are common in dialysis patients. Recommendations related to discrepancies were more likely to be accepted by the providers as compared to MRPs. Medication records became inaccurate within 12 months. A pharmacy-based medication reconciliation and review program may have an important impact on the care of hemodialysis patients.
Key words: Medication Reconciliation; Medication Errors; Renal Dialysis; Interprofessional Relations; Pharmacists; United States.
Introduction
Hemodialysis (HD) is the primary mode of renal replacement therapy for patients with stage 5 chronic kidney disease (CKD) and end-stage renal disease (ESRD). Patients with ESRD can be challenging to manage because they often have multiple co-morbidities and are prescribed an average of 11-12 medications, placing them at an increased risk for adverse events.1 A major obstacle in providing high-quality care to hemodialysis patients is maintaining accurate medication records. In addition, the complex pharmacotherapeutic principles involved in hemodialysis makes the management of these patients challenging. Pharmacists have a unique opportunity to apply their knowledge and skills, thus, improving the care of this patient population.
Medication reconciliation is "the process of creating the most accurate list possible of all medications a patient is taking - including drug name, dosage, frequency, and route - and comparing that list against the physician's admission, transfer, and/or discharge orders, with the goal of providing correct medications to the patient at all transition points within the hospital".2 Many healthcare organizations, driven by various standards and the quest to improve care, have implemented medication reconciliation programs across the spectrum of care for patients. In the general population, there is conflicting evidence regarding the benefits of medication reconciliation. While many discuss its importance for reducing medication errors, adverse events and hospitalization3,4, one large systematic review on the "effectiveness of hospital-based medication reconciliation interventions" failed to find a difference in hospital readmission rates.5
A robust medication reconciliation program may be particularly important for hemodialysis patients as they are at a high risk for medication-record discrepancies. In addition to a long and complex medication regimen, the overall hospitalization rate among patients on dialysis is 1.73 admissions per patient year, much higher than the general population.6 It can also be argued that patients on chronic hemodialysis are in a constant cycle of care transition between home and in-center dialysis. Dialysis patients often see multiple prescribers, which further increases the risk for errors in their medication record. Furthermore, while the medication records maintained in dialysis units are often electronic, they are usually stand-alone systems without integration to the broader health information system. In the event of a hospitalization, the in-center dialysis medication list is often used for admission prescribing. Therefore, errors in the dialysis medication list could be transferred to the in-patient medication regimen, potentially causing any number of medication-related problems (MRP).
As noted by Pai et al., medication reconciliation and medication review are two distinct, yet important functions.1 A medication reconciliation is basically the process of confirming the accuracy of the medication record (usually with the patient or caregiver) and should occur at fairly frequent, defined intervals. A medication review is a much more in depth analysis of the medication regimen including assessing the appropriateness of therapy, dosing, and monitoring of efficacy and side effects.
To conduct a valid medication review, the clinician needs an accurate medication record. Nurses play a critical role in the care of a patient on dialysis and are often the professionals that are responsible for performing medication reconciliations. However, staffing and time constraints in a typical dialysis unit often hinder them from conducting a thorough review. While nurses are able recognize a medication and its dosing frequency, it is a pharmacist's expert knowledge of medication's therapeutic use and adverse effects that allows them to recognize and adjudicate MRPs.7
An MRP is "an event or circumstance involving medication therapy that actually or potentially interferes with an optimum outcome for a specific patient".8 MRPs have a negative effect on patients' morbidity, mortality, and quality of life and are associated with increased healthcare expenditures.9,10 In the general patient population, 59% of hospital admissions due to MRPs are deemed "definitely" or "possibly" avoidable and it is estimated that 100,000 deaths occur annually due to these MRPs.11 On average, the typical hemodialysis patient will experience one MRP per every 6.5 medications they are prescribed.1 MRPs in patients on HD are associated with increased hospitalization. Almost 20% of hospitalizations in patients with stage 5 CKD may be directly related to MRPs.1
Although there is some data on the literature regarding MRPs in dialysis, surprisingly, little information exists on medication record discrepancies. Therefore, we report on the results of a pharmacy-based medication reconciliation and review program in two outpatient dialysis units.
Methods
This study is a prospective chart review with a purpose to: 1) identify the extent and type of medication discrepancies and medication related problems (MRPs) experienced by dialysis patients during pharmacist-initiated medication reviews and 2) determine if the resulting recommendations made by the pharmacy team to the patient's provider were accepted. The protocol was reviewed and approved by an Institutional Review Board and informed consent was obtained from each participant.
A pharmacy-based medication reconciliation and review program has been in place at a regional dialysis unit since 2005. A second unit was added to the program in 2014. The program is intended to 1) reconcile the medication record and 2) identify and resolve MRPs. The pharmacy team consists of a pharmacist/faculty member and second and third year student pharmacists from the Nesbitt School of Pharmacy at Wilkes University. The pharmacy team's role is to conduct one of the required monthly medication reviews that would otherwise be performed by nursing staff at the dialysis unit.
Medication reviews were performed in the two dialysis units. Patients were given a pre-printed note requesting they bring their home medications to the dialysis unit on one of their scheduled days. If patients were reluctant to bring their medications from home, a written or oral list was accepted. The main focus of the patient encounter was medication reconciliation between the patient's medication regimen and the dialysis records. Laboratory values were reviewed for all patients and staff (mostly nursing) was consulted as necessary. Following the medication reconciliation and review, the pharmacy team prepared an updated (corrected) medication list. This list and a brief progress note with any recommendations were provided to the unit staff. Nursing staff updated the medication list and the providers (nephrologists or nurse practitioners) reviewed the note and recommendations related to MRPs.
Subsequent to the patient encounter, any medication record discrepancies and MRPs that were identified were documented on a standardized data collection form (see online supplementary material). Discrepancies were reported similar to that of Leung et al.12 See Table 1 for discrepancy and MRP categorization.
The medical record was reviewed 30 days after the pharmacy reconciliation and review to determine if individual discrepancies were corrected and if MRP recommendations were accepted by the prescriber and/or nursing staff. For any given shift, medication reviews by the pharmacy team were conducted annually. Thirty one patients were seen twice, twelve months apart.
Data collection forms for patient encounters between April 2014 and August 2015 were evaluated. Patients lost to follow up were excluded from the analysis. All patient information was de-identified for the analysis.
Descriptive statistics were used to describe frequencies of discrepancies and MRPs. A chi-squared test was used to compare the proportion of recommendations that were accepted between units. An independent t-test was used to compare the number of discrepancies between the two units. Lastly, a paired t-test was used to compare the number of discrepancies in the 31 patients seen 12 months apart. An a priori level of p<0.05 was used to determine statistical significance. Data are presented descriptively as counts, percentages and mean + standard deviation.
Results
Between April 2014 and August 2015, 124 medication reviews were performed in 93 unique patients (31 patients had a second medication review conducted approximately 12 months apart). Of the 93 original patients, three were lost prior to the 30-day follow up and excluded; one due to death and two because of transfers out of the dialysis unit. In 53% of the reviews, patients brought their medications to the unit. A medication list carried by the patient was used 25% of the time and 22% of the reviews were conducted using the patient's own recollection. Patients were maintained on an average of 11.9 home medications.
During the study period, the pharmacy team made 440 recommendations, of which 376 (85%) were classified as discrepancies and 64 (15%) were classified as MRPs. This equates to 3.1 (SD=2.7) discrepancies and 0.5 (SD=0.8) MRPs per patient. By far, the most commonly identified discrepancies were commission and omission, while indication without drug, drug without indication, and dose too high were the most frequent MRPs (Table 2). In total, 77% of the interventions were accepted by either the prescriber or nurse. MRPs were much less likely to be accepted versus discrepancies (27%, 85% respectively, p<0.001). The top medication classes that were associated with MRPs were: vitamins (20%), statins (19%), phosphate binders (8%), vitamin D analogues (6%), and calcimimetics (5%).
There were a significantly different number of discrepancies and MRPs between the two units (4.4 vs. 2.3 respectively, p=0.002). In addition, the unit with fewer discrepancies per patient was more likely to accept recommendations as compared to the other unit (86% vs. 68% respectively, p<0.001). There was no difference in the number of MRPs per patient, per unit.
The number of discrepancies and MRPs were compared in the 31 patients who underwent a second medication review 12 months later and no significant differences were found between reviews (95 vs 86 discrepancies, 13 vs. 11 MRPs).
Discussion
Our study is unique because we had a relatively large patient population and because we separately evaluated both medication discrepancies and MRPs, and evaluated the results of our recommendations. Our study had a number of important findings which include: 1) pharmacy identified medication discrepancies are common in our dialysis patient population with errors of omission and commission predominating, 2) MRPs were reported less frequently than in other studies1,13 and 3) a pharmacist can have an important impact in the medication reconciliation and review process by correcting the medication record and identifying MRPs.
Surprisingly, there is very little information in the literature regarding medication record discrepancies in dialysis units. Leung et al. also studied medication record discrepancies in hemodialysis patients as identified by pharmacy technicians.12 They found a similar discrepancy rate compared to us (3.8 vs. 3.1 per patient, respectively), but had a much higher rate of undocumented intentional discrepancies (59% vs 5%, respectively).14 This suggests our patients are much less likely to self-manage their medication regimen than the population studied by Leung. Pai et al. listed "drug record discrepancy" as a component of an MRP, which makes quantifying and qualifying discrepancies difficult.15 Furthermore, other studies have been unclear on what constitutes an MRP versus a discrepancy.16
There are a number of reasons why our MRP rate may be lower than other studies. Of course the differences in number of MRPs could be related to a higher quality of patient care in our units but we have no evidence to support this theory. It is important to note that in our study only MRPs that resulted in a recommendation to the prescriber were included. For example, many patients were on an anticoagulant/antiplatelet plus low dose aspirin. This is a common drug-drug interaction and although considered an MRP, we did not claim it as such because in many cases, the prescriber is using these two medications with full knowledge of the risks versus benefits. Another example is the interaction between calcium-based phosphate binders and levothyroxine, in which calcium binds levothyroxine and reduces its absorption. Technically, patients should separate these medications by 4 hours to avoid the interaction. However, if the patient has been taking these two medications together on a routine basis, and they are clinically stable, we did not make an intervention (nor was it documented as an MRP). Similar studies did not state if MRPs of this nature were included or excluded from their studies. Finally, we focused our attention on home medications. The use of an erythropoietin stimulating agent, intravenous iron, and intravenous vitamin D products is highly protocol driven in our units. It is possible that other studies were conducted before the use of protocols was widespread. It is interesting that the study by Manley et al. identified "inappropriate laboratory monitoring" as the most common MRP13, while we had very few (if any) of this type of intervention. Finally, it is possible that student pharmacists are less able to identify MRPs. However, the supervising pharmacist is experienced in the care of hemodialysis patients and reviewed all patient cases with the students.
There are many studies measuring the extent of MRPs in hemodialysis patients, but few quantify whether pharmacy recommendations were implemented by prescribers.13,15,16-18 Kaplan et al. and Tang et al. were the only two studies we found involving HD patients that described a 92% and 76% implementation rate of pharmacist recommendations, respectively.17,18 Possidente et al. found that physicians agreed with 96% of the recommendations made by pharmacists, but it is unclear if the recommendations were actually applied.19 Comparatively, we had a total (reconciliation plus MRP) implementation rate of 77%.
Although 85% of medication record discrepancies identified by us corrected, our ability to resolve MRPs was lower, just 27%. One of the reasons our resolution rate for MRPs may have been relatively small was that the prescriber (nephrologist/nurse practitioner) deemed non-renal-related MRPs as an issue for the primary care physician (PCP) and may not have followed up. In some cases, the pharmacy team would intervene by asking the patient to follow-up with their PCP to resolve any non-renal problem. If the MRP was considered serious enough, the pharmacy team would contact the PCP directly, but this was fairly rare and was not specifically documented.
There were also process-issues that may have influenced the response rate from providers. Anecdotally, we found that sometimes there was miscommunication between nursing and prescriber staff about our recommendations and who was responsible for implementing them.
There are many strengths to our study and study design. Compared to others, our study had a relatively large patient population. We included 121 medication reviews for 90 patients, thereby offering us data on 31 patients with multiple pharmacist points of contact. This gave us the opportunity to assess the longevity of our recommendations in a subset of the study group. In addition, our study was prospective, giving us the ability to follow our recommendations and interventions in real time. Because the study was prospective, we were able to address unsolved discrepancies and/or MRPs at the point of care. More than 50% of the patients brought their medications with them for review and almost 25% of the patients brought a medication list with them, allowing for more accurate and efficient assessments of discrepancies and MRPs. Lastly, we clearly articulated the difference between a medication record discrepancy and MRP. This is important to note as some studies include both as an MRP, or do not specify the difference between the two, thus, affecting how the data is represented.1,15
There are some limitations to our study and study design. As with other studies of medication discrepancies, the assumption is the discrepancy the pharmacist/student identifies is a true discrepancy. Although under supervision of a pharmacy faculty member, the program was mostly student-driven and it is possible that the students missed discrepancies and MRPs. The follow up consisted of a chart review in which we did not interview patients. Thus, it is possible that changes may have been implemented, but if they were not properly documented in the medical record, they would have been missed. Medication adherence is a major obstacle to care, particularly in patients on polypharmacy such as HD patients. Although we always discussed adherence with patients (most often with phosphate binders), we did not document non-adherence as an MRP because we did not have a reliable way to monitor adherence. Finally, our study was not designed to measure the severity of MRPs or important clinical outcomes such as hospitalization, quality of life, or mortality.
Conclusions
Our study found medication record discrepancies are common in our population of hemodialysis patients. Medication discrepancies are problematic in that they may lead to prescribing errors, particularly during transitions of care. In addition, by conducting a second medication reconciliation review 12 months apart, we were able to determine the longevity of our interventions. In the 31 patients studied, there were no differences in the number of medication record discrepancies, suggesting the records became inaccurate within those 12 months. Therefore, medication reconciliation and reviews conducted by a pharmacy team should occur more frequently in hemodialysis patients, but the exact interval has yet to be determined. For various reasons, we found fewer MRPs compared to other studies and MRPs were much less likely to be resolved than medication record discrepancies. Dialysis units should carefully measure the extent of medication record discrepancies and a pharmacy team is in a unique position to provide medication reconciliation and review.
Acknowledgements
The authors wish to thank the following student pharmacists for assistance in medication review and data collection: Courtney Calamia, Motasim Daghash, Tara Engle, Alesha Falzone, Sarah Hassinger, Jesse Kita, Letitia Warunek, and Brittany Wills. The careful review of our manuscript by Dr. Daniel Longyhore is much appreciated.
Conflict of interest
There is no conflict of interest that we should disclose.
Contributions: research idea and study design: EF, NP; data collection/analysis/interpretation: EF, NP; statistical analysis: EF, NP. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. EF takes responsibility that this study has been reported honestly, accurately, and transparently; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
References
1. Pai AB, Cardone KE, Manley HJ, St Peter WL, Shaffer R, Somers M, Mehrotra R; Dialysis Advisory Group of American Society of Nephrology. Medication reconciliation and therapy management in dialysis-dependent patients: need for a systematic approach. Clin J Am Soc Nephrol. 2013;8(11):1988-1999. doi: 10.2215/CJN.01420213. [ Links ]
2. Medication Reconciliation to Prevent Adverse Drug Events. http://www.ihi.org/topics/adesmedicationreconciliation/Pages/default.aspx (accessed September 25, 2015). [ Links ]
3. Jack BW, Chetty VK, Anthony D, Greenwald JL, Sanchez GM, Johnson AE, Forsythe SR, O'Donnell JK, Paasche-Orlow MK, Manasseh C, Martin S, Culpepper L. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med. 2009;150(3):178-187. [ Links ]
4. Schnipper JL, Kirwin JL, Cotugno MC, Wahlstrom SA, Brown BA, Tarvin E, Kachalia A, Horng M, Roy CL, McKean SC, Bates DW. Role of pharmacist counseling in preventing adverse drug events after hospitalization. Arch Intern Med. 2006;166(5):565-571. [ Links ]
5. Kwan JL, Lisha L, Margaret S, Shojania KG. Medication reconciliation during transitions of care as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158(5 Pt 2):397-403. doi: 10.7326/0003-4819-158-5-201303051-00006. [ Links ]
6. Saran R, Li Y, Robinson B, Ayanian J, Balkrishnan R, Bragg-Gresham J, Chen JT, Cope E, Gipson D, He K, Herman W, Heung M, Hirth RA, Jacobsen SS, Kalantar-Zadeh K, Kovesdy CP, Leichtman AB, Lu Y, Molnar MZ, Morgenstern H, Nallamothu B, O'Hare AM, Pisoni R, Plattner B, Port FK, Rao P, Rhee CM, Schaubel DE, Selewski DT, Shahinian V, Sim JJ, Song P, Streja E, Kurella Tamura M, Tentori F, Eggers PW, Agodoa LY, Abbott KC. US Renal Data System 2014 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2015;66(1 Suppl 1):Svii, S1-S305. doi: 10.1053/j.ajkd.2015.05.001. [ Links ]
7. Smith S, Witten B, Paykin C, Weiner S, Chianchiano D, St Peter WL. Medicare Part D: challenges for dialysis patients (part 2 of 2): opportunities to improve patient experiences. Nephrol News Issues. 2011;25(13):24-29. [ Links ]
8. ASHP Statement on Pharmaceutical Care. http://www.ashp.org/DocLibrary/BestPractices/OrgStPharmCare.aspx (accessed September 7, 2015). [ Links ]
9. Ernst FR, Grizzle AJ. Drug-related morbidity and mortality: updating the cost-of-illness model. J Am Pharm Assoc (Wash). 2001;41(2):192-199. [ Links ]
10. Johnson JA, Bootman JL. Drug-related morbidity and mortality. A cost-of-illness model. Arch Intern Med. 1995;155(18):1949-1956. [ Links ]
11. Manley HJ, Barton-Pai A. Integrated pharmacy services: a necessary component for care of patients treated by long-term dialysis. Am J Kidney Dis. 2013;62(3):445-447. doi: 10.1053/j.ajkd.2013.06.004. [ Links ]
12. Leung M, Jung J, Lau W, Kiaii M, Jung B. Best possible medication history for hemodialysis patients obtained by a pharmacy technician. Can J Hosp Pharm. 2009;62(5):386-391. [ Links ]
13. Manley HJ, Cannella CA, Bailie GR, St Peter WL. Medication-related problems in ambulatory hemodialysis patients: a pooled analysis. Am J Kidney Dis. 2005;46(4):669-680. [ Links ]
14. Ong SW, Fernandes OA, Cesta A, Bajcar JM. Drug-related problems on hospital admission: relationship to medication information transfer. Ann Pharmacother. 2006;40(3):408-413. [ Links ]
15. Pai AB, Boyd A, Depczynski J, Chavez IM, Khan N, Manley H. Reduced drug use and hospitalization rates in patients undergoing hemodialysis who received pharmaceutical care: a 2-year, randomized, controlled study. Pharmacotherapy. 2009;29(12):1433-1440. doi: 10.1592/phco.29.12.1433. [ Links ]
16. Manley HJ, Drayer DK, Muther RS. Medication-related problem type and appearance rate in ambulatory hemodialysis patients. BMC Nephrol. 2003;4:10. [ Links ]
17. Tang I, Vrahnos D, Hatoum H, Lau A. Effectiveness of clinical pharmacist interventions in a hemodialysis unit. Clin Ther. 1993;15(2):459-464. [ Links ]
18. Kaplan B, Shimp LA, Mason NA, Ascione FJ. Chronic hemodialysis patients. Part II: Reducing drug-related problems through application of the focused drug therapy review program. Ann Pharmacother. 1994;28(3):320-324. [ Links ]
19. Possidente CJ, Bailie GR, Hood VL. Disruptions in drug therapy in long-term dialysis patients who require hospitalization. Am J Health Syst Pharm. 1999;56(19):1961-1964. [ Links ]
Received (first version): 22.04.16
Accepted: 4.08.16