Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Nutrición Hospitalaria
versión On-line ISSN 1699-5198versión impresa ISSN 0212-1611
Nutr. Hosp. vol.20 no.6 Madrid nov./dic. 2005
Original
Ineffectiveness of Nickel in augmenting the hepatotoxicity in protein deficient rats
P. Sidhua, M. L. Gargb, P. Morgensternc, J. Vogtd, T. Butzd, D. K. Dhawana*
aInstitute of Physiology and Experimental Pathophysiology ,Friedrich-Alexander University,Erlangen-91054,Germany.
bDepartment of Biophysics, Panjab University, Chandigarh-160014, India. cUmweltforschungszentrum Leipzig-Halle, Leipzig,
Germany für Physik und Geowissenschaften,Universität Leipzig, Leipzig,Germany.dFakultät für Physik und
Geowissenschaften,Universität Leipzig, Leipzig,Germany. *Department of Biophysics Panjab University Chandigarh, India..
| Abstract This study was designed to determine the toxic effects of nickel sulfate on the biochemical and elemental profile of liver in protein deficient rats. Nickel sulfate in the dose of 800mg/l in drinking water was administrated to Sprauge Dawley (S.D) normal control as well as protein deficient rats for a total duration of eight weeks. The effects of nickel treatment and protein deficiency when given separately and in combination were studied on rat liver marker enzymes like Alkaline phosphatase (ALP),Glutamate oxaloacetate transaminase (GOT), Glutamate pyruvate transaminase (GPT) and also on the (Nutr Hosp 2005, 20:378-385) Key words: Nickel. Protein deficiency. Liver marker enzymes. Trace elements. | EFICACIA DEL NÍQUEL EN AUMENTAR LA HEPATOTOXICIDAD EN RATAS CON DEFICIENCIA EN PROTEÍNAS Resumen Este estudio fue diseñado para determinar los efectos tóxicos del sulfato de níquel sobre el perfil bioquímico y de oligoelementos del hígado en ratas con deficiencia de proteínas.Se administró sulfato de níquel, a la dosis de 800 mg/l, en el agua de bebida de ratas Sprauge Dawley (S-D) normales control y con deficiencia de proteínas, durante 8 semanas. Se estudiaron los efectos del tratamiento con níquel y de la deficiencia de proteínas, por separado y en combinación,sobre marcadores enzimáticos hepáticos de la rata como la fosfatasa alcalina (FA), la glutamato oxalacetato transaminasa (GOT), la glutamato piruvato transaminasa (GPT) y también el estado de oligoelementos en el hígado de la rata. Las ratas con deficiencia de proteínas, las ratas tratadas con níquel, así cómo aquéllas con la combinación de deficiencia de proteínas y tratamiento con níquel mostraron reducciones significativas en el peso corporal y en el contenido hepático de proteína, en comparación con las ratas normales control. La actividad hepática fosfatasa alcalina y alanina aminotransferasa mostró una elevación significativa en las ratas sometidas a deficiencia de proteínas, a tratamiento con níquel, y a la combinación de deficiencia de proteínas y tratamiento con níquel. Con respecto de las concentraciones hepáticas de aspartato aminotransferasa, se observó una elevación significativa en los animales con deficiencia de proteínas y en aquellos tratado con níquel y con deficiencia de proteínas. La administración de níquel a ratas normales y con deficiencia de proteínas ha producido un aumento significativo de las concentraciones de níquel, fósforo y azufre en el tejido hepático. La concentración de cinc y cobre en el tejido hepático disminuyó significativamente los animales con deficiencia de proteínas, los tratados con níquel, y aquellos con deficiencia de proteínas tratados con níquel. Se halló que las concentraciones tisulares de hierro estaban disminuidas en los animales con deficiencia de proteínas, pero aumentaron significativamente en los animales tratados níquel y aquellos con deficiencia de proteínas tratados con níquel. Se observó que el selenio disminuyó significativamente en los animales con deficiencia de proteínas, los tratados con níquel, y aquellos con deficiencia de proteínas tratados con níquel, en comparación con los animales normales. La elevación de selenio en los animales con deficiencia de proteínas tratados con níquel también fue significativamente superior en comparación con los animales con deficiencia de proteínas. (Nutr Hosp 2005, 20:378-385) Palabras clave: Níquel. Deficiencia de proteínas. Enzimas marcadoras hepáticas. Elementos traza. |
Correspondencia: P. Sidhu
E-mail: pardeepsidhu@yahoo.co.ur
Recibido: 18-X-2004.
Aceptado: 6-III-2005.
Introduction
In the developing countries like India protein nergy malnutrition (PEM), is a prominent cause of malnutrition and this may be associated with adverse functional disorders of body metabolism, which is likely to be exaggerated in conditions of heavy metal toxicity. Malnourished people living in the vicinity of nickel (Ni) industrial and smelting units are more prone to nickel toxicity. The protein calorie malnutrition affects the synthesis of the mRNA suggesting that a defect occurs at a pre-transcriptional level that results in a diminution of the concentration of mRNA1. PEM is known to be associated with many biochemical disturbances in the body. The increases in serum alanine aminotransferease and alkaline phosphatase in animals fed on a protein deficient diet, suggests hepatocellular dysfunctions2, 3.
Persons afflicted with protein malnutrition are almost deficient in a variety of micronutrients. Protein deficiency has been shown to decrease the hepatic levels of zinc, manganese, copper, calcium and magnesium in experimental animals4, 5. Alterations in the levels of trace elements results in number of diseases like hypoalbuminemia, and anemia in malnourished children6. Bhaskaram and Hemalatha, 19957 observed that children suffering from severe protein energy malnutrition have very low levels of the thymulin hormone, which is a sensitive indicator of zinc status in body. It is known that dietary protein variation affects the absorption and uptake of 65Zn8.
Nickel is a common respirable sized particulate pollutant and nickel compounds of commercial importance are used in the preparation of nickel alloys, ground coated enamels, in cooling of ceramics and glass, electroplating, nickel-iron storage batteries, electronic components and to prepare nickel catalysts9. Nickel breaks down the immunity by affecting the T-cell system and suppresses the activity of natural killer cells in rats and mice10, 11.
Nickel has been shown to interact with number of trace elements including iron, zinc, copper, manganese, sodium and potassium12, 13. Nickel mobilizes and promotes the excretion of copper, zinc and manganese from organs and promotes storage of chromium in organs14. Nickel affects the iron metabolism and intensifies the erythropoiesis with induced hemolytic anemia15.
In view of the increasing incidences of cancer, cardiovascular, respiratory and gastrointestinal diseases, due to heavy metal toxicity, the analysis of dietary intake for the toxic and protective elements and their levelsin tissue needs further research. Since, nutrients are essential for all the fundamental cellular processes as they modulate cellular oxidative and antioxidativedefense system in the body, therefore, it becomes a matter of concern to elucidate their role in manifestation of functional disorders of different organs.
Liver, being a major metabolic organ, plays an important role in the regulation of biochemical and trace element metabolism. The present investigations were designed to study the hepatotoxic effects of nickel in rats subjected to protein deficiency.
Materials and methods
Grouping of animals
Rats in the weight range of 110-120 g of Sprague Dawley (S.D.) strain were procured from the Central Animal House, Panjab University, Chandigarh. The animals were housed in polypropylene cages in the animal house of the Department of Biophysics, under hygienic conditions and were acclimatized for at least one week before putting them on different treatments. Thereafter, the animals were randomly and equally divided into the four groups each having ten animals.
G-1, Normal Control
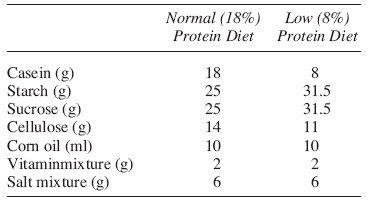
Animals in this group served as normal controls and were fed with diet containing normal protein contents (18%). Composition of the diet used was as described by Kaur et al, 1992 and given in table below.
G-2, Protein deficient,(PD)
Protein deficiency was induced in the animals of this group by maintaining them on the laboratory prepared protein deficient diet with 8% protein contents. Composition of the diet used was as described by Kaur et al, 1992 (16) and given in table below.
G-3, Nickel treated, (Ni)
Animals in this group were given nickel in the form of NiSO4. 6H2O at a dose level of 800 mg/L in drinking water and the animals had free access to the drinking water containing nickel and the normal diet.
G-4, Ni+PD treated
Animals in this group were given protein deficient diet as given to G-2 animals and in addition were subjected to Ni treatment as mentioned for G-3 animals.
Composition of diets (weight %)
The composition of the diet is given in the following table (Kaur y cols., 1992).
The treatments of rats continued for a period of eight weeks. At the end of the treatment, the animals were weighted and were sacrificed by exsanguination under light anesthesia. Livers were removed immediately and were perfused and rinsed in normal saline (NaCl, 9 g/l/w/v). One lobe was preserved by freezing for the determination of various trace elements and the other was processed immediately for various biochemical studies.
Biochemical Estimations.
Protein
Protein assay was done by the method of Lowry y cols., 195117.
Estimation of liver marker enzymes in liver
The enzyme activity of Alkaline Phosphatase (ALP) was measured by the method of Wooton18 and the enzyme activities of aspartate aminotransferases (AST) and Alanine Aminotransferase (ALT) were estimated according to the procedure of Reitman and Frankel19.
Elemental analysis of liver samples
Estimations of various elements in the liver samples of different treatment groups were carried using Energy Depressive X-ray Fluorescence (EDXRF) technique. Energy depressive X-ray fluorescence technique is one of the most suitable analytical method to analyze trace elements because of its properties such as non-destructive, sensitivity up to ppm and multi-elemental analysis.
- Sample Preparation for EDXRF
The liver tissues of all the animals were oven dried at 70ºC to a constant weight and then ground with the help of Agate Pestle and Mortar. 300 mg dried powder of the tissue so obtained was weighed and mixed with equivalent amount of Hoechst Wachs (wax) to make self supporting pellets. The pellets were made by using a specially designed pure steel dye and a hydraulic press from Paul weber, Germany. A force of approximately 45 KN (Kilo newtons) was applied at the dye top in order to make pellets of uniform thickness.
- EDXRF Setup
In the present work, the pellets of tissues were analyzed using an EDXRF X-Lab, 2000 to determine the levels of various elements. The X-lab, 2000 spectrometer involved a 0.4 kw Pd anode X-ray tube as source of excitation. The power of the X-ray tube was adjusted on line for each individual measurement by the spectropho-tometer software, to secure optimum acquisition parameters for the current analysis.
Presently, different X-ray energies and excitation modes are being used but the most important mode used was of 40 kV and excitation used was polarized X-Ray. A Si (Li) detector coupled with computer (Pentium, 600 MHz, software package SPECTRO X-LABPRO 2.2) was used to collect the flourescent X-ray spectra from the samples. The X-ray tube, secondary exciter, target and the Si (Li) detector were placed in a triaxial geometry mode. This geometry was used to minimize the background due to scattered photons.
Results
The results of all the experiments conducted during the current study are depicted in various tables. All the results of various treatment groups have been compared with their normal controls. Results of nickel + protein deficient (G-4) treated group have been compared with the results of the protein deficient group (G-2) also.
Statistsical Analysis
The statistical significance of the values has been determined by using one way analysis of variance (ANOVA) followed by Student-Newman-Keuls test. The determinations are represented as Mean ± SD.
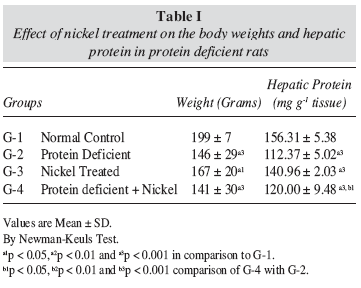
Body weights
The variations in the body weights of the animals subjected to different treatments are shown in table I. It was observed that the protein deficiency resulted in a significant (p < 0.001) decease in the body weights after eight weeks, when compared to normal control rats. Nickel treatment to normal control rats resulted in some decrease (p < 0.05) in the body weights but nickel treatment to protein deficient rats resulted in appreciable reduction (p < 0.001) in the body weights as compared to normal control rats.
Hepatic protein Contents
The hepatic protein contents in various treatment groups expressed as mg g-1 tissue are shown in tableI. Protein deficient, Ni treated as well as combined protein deficient and nickel treated rats showed significant (P < 0.001) reductions in the hepatic protein contents as compared to normal control rats.
Alkaline phosphatase
Hepatic alkaline phosphatase activity showed a significant elevation (p < 0.01) in rats subjected to protein deficiency, nickel treatment and combined protein deficiency and nickel treatment as shown in table II.
Aspartate Aminotransferase
As regards to hepatic levels of AST, a significant (p < 0.001) elevation was observed in protein deficient and nickel treated protein deficient animals (table II).
Alanine Aminotransferase
Table II depicts the hepatic observations of ALT where significant (p < 0.001) elevation in ALT levels has been observed in protein deficient, nickel and nickel treated protein deficient animals.
Hepatic concentration of various elements
The concentrations of various elements have been depicted in table III. The nickel administration to normal and protein deficient rats has resulted in a significant (p < 0.001) increase in concentrations of nickel in liver tissue.
The concentration of zinc and copper in liver tissue got decreased significantly (p < 0.001) in protein deficient, nickel treated and nickel treated protein deficient animals.
Tissue iron concentrations were found to be decreased in protein deficient animals, but the concentrations of iron got elevated significantly (p < 0.001) in nickel treated and nickel treated protein deficient animals.
It has been observed that selenium got decreased significantly (p < 0.001) in protein deficient, nickel treated and nickel treated protein deficient animals when compared to normal animals. The elevation of selenium in nickel treated protein deficient animals was also significantly (p < 0.05) higher when compared to protein deficient animals.
Significant (p < 0.001) decrease has been observed in potassium concentration in nickel treated and nickel treated protein deficient animals. On the other hand phosphorus and sulfur concentrations were found to be increased significantly in nickel treated and nickel treated protein deficient animals.
Discussion
We observed a significant (p < 0.001) decline in the body weights of rats subjected to protein deficiency for a period of eight weeks, when compared to normal control rats. Loss in body weight is characteristic of protein malnutrition. In an earlier report from our laboratory it has been observed that protein deficiency leads to significant growth retardation in animals2. Many other workers have also reported the decrease in body weight due to protein deficiency20, 21. It has been seen in these reports that retardation in body weight growth over a period is not due to low intake of diet but deficiency in protein intake.
Nickel treatment to normal control and protein deficient rats resulted in marked reduction in the body weights as compared to normal control rats. The reduction in body weights following nickel treatment has also been reported earlier22, 23. The decrease in body weight may not solely be attributed to protein deficiency alone as the Ni treatment alone also has caused significant decline in body weight. The decrease in body weight due to nickel treatment has been connected by researchers to be not due to low intake of diet consumption of the rats following toxic treatment with nickel, vis a vis normal rats, and thus it is anticipated that this effect could possibly be due to the overall increased degeneration of lipids proteins as a result of nickel toxicity23,24.
Rats in protein deficient, Ni treated and combined PD+Ni treated groups, showed a highly significant (p < 0.001) reduction in the hepatic protein contents as compared to rats of normal control group. Davenport y cols., 199425 demonstrated that in protein deficient states, the reduction in protein contents are due to depletion in amino acid precursors. Nickel in earlier reports, has also been able to cause significant depression in protein levels26,27. Nickel diminishes the DNA and RNA polymerase activity and decreases DNA replication fidelity28 which in turn can reduce the protein synthesis.
Hepatic alkaline phosphatase activity folowed a significant elevation due to protein deficiency and nickel treatment. This elevation coul be anticipated to the reason that ALP is bound to the intracellular membranes, and does not leak out with the increased permeability of the cell membranes. Moreover, Hultberg and Disaksson, 198329 proposed that activated macrophages including the Kupffer cells are the cellular source for the increased levels of ALP in conditions of liver damage. Davenport y cols., 199425 also postulated tath many hepatic and extrahepatic conditions could also result due to protein-restricted diets that in a way caused increased production of alkaline phosphatase isoenzymes from bone and hepatobiliary source.
The aminotransferases are intracellular enzymes, which are active in operating the reversible exchange of aminoacids between alpha-amino and alpha-ketoacids. As all the naturally occurring amino acids can undergo amino transfer reactions thus this class of intracellular enzymes (aminotransferases) form an important link between protein and carbohydrate metabolism30.
Hepatic levels of AST and ALT got significantly (p<0.001) elevated in protein deficient and nickel treated protein deficient animals. The observed increased activities of hepatic AST and ALT in the animals given protein deficient diet are in conformity with the study of Pond y cols., 199231. They reported that the increase in ALT activity in protein deficient pigs shows altered liver functions, although microscopic anatomy revealed no evidence of excessive fat accumulation or of pathologic changes. Similar observations were noticed by Kumari y cols., 199332 who estimated ALT and AST activities in the samples of 30 cases of pediatric PEM. These authors explained that during hepatobiliary disorder, amino acids are released form exaggerated tissue breakdown and in order to metabolize these amino acids, the process of transmutation gets enhanced leading to increased activity of the related enzymes AST and ALT. Heavy metals have also been found to cause the increase in AST and ALT levels33. A earlier report from this laboratory has also shown the alterations in the AST and ALT concentrations due to lead intoxication34.
It is now well authorized that the liver has an important function in the regulation of trace element metabolism35, 36. Further trace elements serve as cofactors for many enzymes in numerous metabolic pathways; therefore, changes in the distribution of these essential elements in the body can have both nutritional and toxicological consequences with regard to the metabolism of other metals37. Those metals which are essential for maintenance of the structural and functional integrity of the living organisms are found in all living systems and are conserved within strict concentration limits in the systems38. However, imbalances in the supply of any of the essential elements in the body can have both nutritional and toxicological consequences with regard to the metabolism of other metals. They can further be responsible for the development of clinical signs of trace element deficiencies or can modify the susceptibility to metal toxicity39. It is well insinuated that metals that have similar chemical and physical properties would often interact biologically and antagonize or embellish each other's function34.
In the present study, nickel concentration has been found to be increased in liver tissue following the administration of nickel to normal and protein deficient rats. Our results are in agreement with earlier reports40, 41.
Although zinc had been known to be essential for the growth of microorganisms for over hundred years, the advances in the knowledge of zinc chemistry and biochemistry have been explored only during the last two decades42. Furthermore, a large number of zinccontaining enzymes and proteins have been recognized to participate in the metabolism of proteins, nucleic acids, carbohydrates and lipids. Consequently, zinc deficiency is the most significant pathological and biochemical state involving abnormalities in the metal's metabolism. This can be due to inadequate dietary intake, increased requirements or excretion, conditioned deficiency or genetic disorders43, 44.
In present study, observation of depressed Zn levels in liver of protein deficient rats are in conformity with previous studies45, 46. We have also observed decrease in hepatic zinc concentration following nickel toxicity, which may be because nickel mobilizes and promotes the excretion of copper, zinc and manganese from organs and promotes storage of chromium in organs14, 23. Abnormalities in zinc metabolism leading to its deficiency are generally attributed to various factors like, malabsorption, malnutrition, decreased intestinal zinc binding factors or the increased excretion of the zinc via the gastrointestinal tract or via urine are of common occurrence in chronic liver disorders47.
Protein restricted diet led to marked reduction in copper contents in the present study which is in agreement with earlier reports45, 46. Copper depletion is associated with depressed hepatic Cu-Zn superoxide dismutase (SOD) activity and it is well known that protein deficiency results in reduction in SOD activity48. Since Ni and Cu are closely related chemically, an interaction between these two metals may exist. Significant inhibition is observed in copper concentrations, following nickel and protein deficient treatment. High levels of dietary Ni have been reported to decrease the levels of tissue copper and activities of a Cucontaining enzymes like cytochrome oxidase49. In an earlier study from our lab, hepatic Cu levels have also been found to be decreased following lead treatment50.
Iron plays an important role, not only in oxygen delivery to the tissues, but also as a cofactor with several enzymes involved in energy metabolism and thermoregulation51. Further, the enzymes cytochromes P450 and b5 involve the metal in the metabolism of compounds such as steroids and in the degradation of xenobiotics52, 53.
The hepatic Fe contents, in the present study, were found significantly depressed in protein deficient rats as compared to normal control group. Reduction in Fe contents following protein deficiency has previously been reported by Klavins et al, 196254 and Tandon y cols., 19994. They concluded that protein plays an important role in the absorption of iron from the gastrointestinal tract and protein deficiency results in decreased absorption of iron.
During the course of this study, we have also observed increased hepatic iron contents in the animals, given nickel treatment, which has also been reported earlier55. Pronounced increase in iron concentration at a higher dose of nickel could be because of nickel functioning as a cofactor that facilitates the intestinal absorption of the iron by enhancing its compellation to a lipophilic molecule56. Increased hepatic iron levels in response to the toxic conditions established by nickel also suggests that the body requirement of iron has been so severe that more and more of this metal ion is transported to the liver, as it serves as a cofactor for many key enzymes involved in various energy generation pathways, which could have been adversely affected in stress conditions. When serum iron levels exceed the iron binding capacity of transferrin a β1- glycoprotein synthesized in the liver, the circulating free iron initially accumulates in Kupffer cells and later in the hepatocytes resulting in the increased concentration of iron in liver tissue. It can also be speculated that the nickel treatment leads to the activation of host defense system which results in the enhancement of hepatic iron content so as to combat the toxic conditions created by nickel administration. Another possible reason for increased liver iron concentrations could be thought of due to impaired hepatic elimination of this metal from the liver. Hepatic iron overload as observed in our study may lead to the development of a severe oxidative stress status in the tissue, thus contributing to the concomitant liver injury as reported by Boisier y cols., 199957 and also observed by us in the form of enhanced ALP, AST and ALT levels.
Selenium, which is an essential trace metal and inactivates sulfhydryl groups in certain enzymes and is also a component of glutathione peroxidase58. This ubiquitous enzyme located in both cytosol and mitochondrial matrix uses glutathione to reduce organic hydropero-xides, thereby, prevents oxidative damage to various cell organelles. In the present study, selenium was found to be decreased significantly in protein deficient, nickel treated and nickel treated protein deficient animals. It has been reported that nickel and selenium act antagonistically and the detoxifying effect of selenium on nickel toxicity seems to be due to the formation of a Ni-selenide excretable complex59 which seems to have been marginalized due to high dose of nickel in the present study.
Potassium is an essential intracellular ion involved in cellular homeostasis and electrical conduction. We have observed a statistically significant decrease in potassium levels in nickel treated and nickel treated protein deficient animals. Rai y cols.,199012 has also observed the loss of K+ and Na+ by Nickel. As potassium is a major cation of intracellular fluid, and functions in balance with the extracellular ionized sodium to maintain normal osmotic pressure and water balance hence it is possible that nickel could have lead to alterations in the membrane permeability of hepatocytes especially with regard to potassium channels or has caused inhibition of ATPase leading to decreased levels of potassium. Its deficiency affects the activity of muscles and transmission of electrochemical impulses. It has been reported that nickel inhibits the Na-K-ATPase60.
Phosphorus, which is an essential part of key cellular nucleoproteins such as DNA, has also been found to increase significantly in nickel treated and nickel treated protein deficient animals. It may possibly be due to increased requirement of phosphorus either due to the inhibitory effects of nickel on DNA synthesis leading to hyperplasia or increased mobilization from bones. Sulfur is an important constituent of many amino acids like methionine, cysteine, cystine, homocysteine, homocystine, and taurine and also of enzymes like S-adenosylmethionine (SAM) and glutathione (GSH)61. Sulfur concentrations got elevated in nickel and PD+Ni treated animals, which could be to counter the toxic effects of nickel.
Our results in the present findings indicate the disturbance in the marker enzymes of rat liver following protein deficiency (PD) and nickel treatment which may be the consequence of alterations in the levels of essential trace elements as a result of hepatic injury.
Conclusion
The findings indicate that protein deficiency does not enhance the signs of nickel toxicity in rats.
Acknowledgements
This work was supported by grant from IUC-DAE, Kolkata and ICMR, New Delhi.
References
1. Adames AE, Requena CR, Pascale JM and Adames M: Effects of maternal protein-calorie malnutrition on the concentration of protein A messenger RNA in surfactant of fetal rat lungs. Rev Med Panama 1999; 24(1):26-33. [ Links ]
2. Tandon A, Dhawan DK and Nagpaul JP: Effect of lithium on hepatic lipid peroxidation and antioxidative enzymes under different dietary protein regimens. J Appl Toxicol 1998;18:187-90. [ Links ]
3. Obatolu VA, Ketiku A, Adebowale EA: Effect of feeding maize/ legume mixtures on biochemical indices in rats. Ann Nutr Metab 2003; 47(3-4):170-5. [ Links ]
4. Tandon A, Nagpaul JP, Bandhu H, Singh N, Dhawan D: Effect of lithium on hepatic and serum elemental status under different dietary protein regimens. Biol Trace Elem Res 1999; 68:51-62. [ Links ]
5. Squali Houssaini FZ, Foulon T, Payen N, Iraqi MR, Arnaud J y Groslambert P: Plasma fatty acid status in Moroccan children: increased lipid peroxidation and impaired polyunsaturated fatty acid metabolism in protein-calorie malnutrition. Biomed Pharmacother 2001; 55(3):155-62. [ Links ]
6. Singla PN, Chand P, Kumar A y Kachhawaha JS: Serum, zinc and copper levels in children with protein energy malnutrition. Indian J Pediatr 1996; 63(2):199-203. [ Links ]
7. Bhaskaram P and Hemalatha P: Zinc status of Indian children Indian J Med Res 1995; 102:210-5. [ Links ]
8. Babcock AK and Henkin RI: Effects of oral zinc loading on zinc metabolism in human II. In vivo kinetics. Metab Clin Exp 1982; 31:335-47. [ Links ]
9. Denkhaus E and Salnikow K: Nickel essentiality, toxicity, and carcinogenicity. Crit Rev Oncol Hematol 2002; 42(1):35-56. [ Links ]
10. Goutet M, Ban M and Binet S: Effects of nickel sulfate on pulmonary natural immunity in Wistar rats. Toxicology 145(1): 15-26. [ Links ]
11. Condevaux F, Guichard J, Forichon A, Aujoulat M y Descotes J: Compared effects of morphine and nickel chloride on NK cell activity in vitro in rats and monkeys. J Appl Toxicol 2001; 21(5):431-4. [ Links ]
12. Rai LC, Raizada M, Mallick N, Husaini Y, Singh AK y Dubey SK: Effect of four heavy metals on the biology of Nostoc muscorum. Biol Met 1990; 2(4):229-34. [ Links ]
13. Alcon MP, Arola L and Mas A: Response to acute nickel toxicity in rats as a function of sex. Biol Met 1991; 4(3):136-40. [ Links ]
14. Henry AS, Marian M and Alexis PN: Life term effects of nickel in rats: Survival, Tumors, Interactions with trace elements and tissue levels. J Nutr 1974; 104:239-43. [ Links ]
15. Anke M, Kronemann H, Grappel B, Hening A, Meissner D and Schneider HJ: The influence of Ni deficiency on growth, reproduction, longevity and different biochemical parameters of goats In: 3, Supeenelement Symposium Nickel. pp. 3-10, Friedrich Schiller Universtat, Jena DDR. [ Links ]
16. Kaur J, Jaswal VM, Nagpaul JP, Mahmood A: Chronic ethanol feeding and microvillus membrane glycosylation in normal and protein-malnourished rat intestine. Nutrition 1992; 8(5):338-42. [ Links ]
17. Lowry OH, Rosebrough NJ, Farr AL and Randall J: Protein measurement with the Folin Phenol reagent. J Biol Chem 1951; 193: 265-75. [ Links ]
18. Wooton IDP: In: Microanalysis in Medical Biochemistry (4th edition, King, E.J., Churchill, London), 1964; 101-5. [ Links ]
19. Reitman S y Frankel S: A colorimetric method for the determination of serum glutamic oxaloacetic acid and glutamic pyruvic transaminases. Am J Clin Pathol 1957; 28:56. [ Links ]
20. Wang G, Yu S and Bao C: Effect of different levels of protein intake on metabolism of protein, zinc and copper in rats. Zhonguo Gonggong Weishing Xuebao. 1995; 14 (2): 90-3. [ Links ]
21. Das KK and Dasgupta S: Effect of nickel sulfate on testicular steroidogenesis in rats during protein restriction. Environ Health Perspect 2002; 110(9):923-26. [ Links ]
22. Sunderman FW Jr, Shen SK, Mitchell JM, Allpass PR y Damjanov I: Embryotoxicity and fetal toxicity of nickel in rats. Toxicol Appl Pharmacol 1978; 43(2):381-90. [ Links ]
23. Cempel M and Janicka K: Distribution of nickel, zinc, and copper in rat organs after oral administration of nickel(II) chloride. Biol Trace Elem Res 2002; 90(1-3):215-26. [ Links ]
24. Dieter MP, Jameson CW, Tucker AN, Luster MI, French JE, Hong HL, Boorman GA: Evaluation of tissue disposition, myelopoietic, and immunologic responses in mice after longterm exposure to nickel sulfate in the drinking water. J Toxicol Environ Health 1988; 24(3):357-72. [ Links ]
25. Davenport DJ, Mostardi RA, Richardson DC, Gross KL, Greene, KA, Blair K: Protein-deficient diet alters serum alkaline phosphatase, bile acids, protein and urea nitrogen in dogs. J Nutr 1994; 124: 2677S-79S. [ Links ]
26. Sreedevi P, Sivaramakrishan B, Suresh A, Radhakrishanaiah K: Effect of nickel on some aspects of protein metabolism inselected organs of the freshwater mussel Lamellidens marginalis.Biomed Environ Sci 1992; 5(3):208-20. [ Links ]
27. Kusal KD and Shakuntala D: Effect of Ni on testicular nucleic acid concentration on protein restriction. Biol Trace Elem Res2000; 73(2):175-180. [ Links ]
28. Sirover MA and Loeb LA: Infidelitly of DNA synthesis in vitro; screening for potential metal mutagens carcinogens. Science 1976; 194:1434-36. [ Links ]
29. Hultberg B, Isakasson A: Isoenzyme Pattern of serum B- Hexosaminidose in liver disease, Alcohol intoxication and pregnancy. Enzyme 1983; 30:166-71. [ Links ]
30. Plaa GL and Hewitt WR: Detection and evaluation of chemically induced liver injury. In: Principles and methods of toxicology. (Eds. Hayes, W. A., Raven Press Ltd., New York), 1989; 599-628. [ Links ]
31. Pond WC, Ellis KJ, Schoknecht P: Response of blood serum constituents to production of and recovery from a kwashiorkor-like syndrome in the young pig. Proc Soc Exp Biol Med 1992; 200:555-61. [ Links ]
32. Kumari R, Rao YN, Talukdar B, Agarwal S, Puri RK: Serum enzymes abnormalities in protein energy malnutrition. Indian Pediatrics 1993; 30:469-73. [ Links ]
33. Bersenyi A, Fekete SG, Szocs Z, Berta E: Effect of ingested heavy metals (Cd, Pb and Hg) on haematology and serum biochemistry in rabbits. Acta Vet Hung. 2003; 51(3):297-304. [ Links ]
34. Singh B, Dhawan D, Chand B y cols.: Lead pollution-its impact on the status of other hepatic trace metals in blood and alteration in the hepatic functions. Biol Trace Elem Res 1994; 40:21-9. [ Links ]
35. Failla ML, Kiser RA: Hepatic and renal metabolism of copper and zinc in the diabetic rat. Am J Physiol 1983; 244:E113-21. [ Links ]
36. Dhawan D, Goel A: Further evidence for zinc as a hepatoprotective agent in rat liver toxicity. Exp Mol Pathol 1996; 63: 110-17. [ Links ]
37. Dhawan DK and Goel A: Protective role of zinc on rat liver function in long term toxicity induced by carbon tetrachloride. J Trace Elements In Experimental Medicine 1994; 7:1-9. [ Links ]
38. McCall JT, Goldstein NP and Smith LH: Implications of trace metals in human diseases. Fed Proc 1971; 30 1011-15. [ Links ]
39. Hill CH and Matrone G: Chemical parameters in the study of in-vivo and in-vitro interactions of various trace elements. Fed Proc 1970; 29:1474-81. [ Links ]
40. Severa J, Yskocil, Fiala Z and Cizara M: Distribution of nickel in body fluids and organs of rats chronically exposed to nickel sulphate. Human and Experimental Toxicology 1995; 14:955-58. [ Links ]
41. Obone E, Chakrabarti SK, Bai C, Malick MA, Lamontagne L, Subramanian KS: Toxicity and bioaccumulation of nickel sulfate in Sprague-Dawley rats following 13 weeks of subchronic exposure. J Toxicol Environ Health 1999; 57(6):379-401. [ Links ]
42. Vallee BL, Falchuk KH: The biochemical basis of zinc physiology. Physiol Reviews 1983; 73(1):79-118. [ Links ]
43. Forbes RM: Use of laboratory animals to define physiological functions and bioavailability of zinc. Fed Proc 1984; 43:2835-39. [ Links ]
44. Vallee BL, Galden A: The metallo-biochemistry of Zn enzyme. Advances In Enzymology 1984; 56:284-430. [ Links ]
45. Wallwork JC, Johnson LK, Milne DB, Sanstead HH: The effect of interaction between dietary egg white protein and zinc on body weight , bone growth and tissue trance metals in the 30 day old rat. J Nutr 1983; 113:1307-20. [ Links ]
46. Martel AC, Reimers EG, Fernández FS, Aleman VC, Martin LG, Moreno FR, Riera AM (1992). Combined effects of ethanol and protein deficiency on hepatic iron, zinc, manganese and copper contents . Alcohol 1992; 9:341-48. [ Links ]
47. McClain CJ and Su Le: Zinc deficiency in the alcoholic alcoholism. Winters Press, New York, 1983; 7:5-10. [ Links ]
48. Huang CJ, Fwu ML: Degree of protein deficiency affects the extent of the depression of ant oxidative enzyme activities and the enhancement of tissue lipid peroxidation in rats. J Nutr 1993; 123:803-10. [ Links ]
49. Burnett FM: A possible role of zinc in the pathology of dementia. Lancet 1981; 1:186-188. [ Links ]
50. Dhawan D, Singh B, Chand B, Singh N, Mangal PC, Trehan PN: X-ray flourescence in the assessment of inter elemental interactions in rat liver following lead treatment. Biometals 1995; 8:105-10. [ Links ]
51. Rosenzweig PH, Volpe SL: Iron, thermoregulation, and metabolic rate Crit Rev Food Sci Nutr 1999; 39(2):131-48. [ Links ]
52. Galan P, Hereberg S, Tovitouy Y: The activity of tissue enzyme in iron deficient rats and man; an overview. Comparative biochemistry and physiology 1984; 77(4):647-53. [ Links ]
53. Aggette PJ: Physiology and metabolism of essential trace elements. Clinics in endocrinology and metabolism 1985; 14(3):281. [ Links ]
54. Klavins JV, Kineey TD, Kaufman N: The influence of dietary protein on iron absorption. Br J Exp Pathol 1962; 43:172-80. [ Links ]
55. Forrest HN, Thomas JZ, Michael EC, Duane RM: Nickel Deprivation in Rats: Nickel-Iron Interactions. J Nutr 1979; 109:1623-32. [ Links ]
56. Nielsen FH, Shuler TR, Mcleod TG, Zimmerman TJ: Nickel influences iron metabolism through physiologic, pharmacologic and toxicologic mechanism in the rat. J Nutr 1984; 114: 1280-88. [ Links ]
57. Boisier X, Schon M, Sepulveda A, Basualdo A, Cornejo P, Bosco C, Carrion Y, Galleano M, Tapia G, Puntarulo S, Fernandez V, Videla LA: Dearrangement of Kupffer cell functioning and hepatotoxicity in hyperthyroid rats subjected to acute iron overload. Redox Rep 1999; 4(5):243-50. [ Links ]
58. Rukgauer M, Neugebauer RJ, Plecko T: The relation between selenium, zinc and copper concentration and the trace element dependent antioxidative status. J Trace Elem Med Biol 2001;15(2-3):73-8. [ Links ]
59. Khandelwal S, Flora SJ and Tandon SK: Nickel-selenium interaction- time dependent biochemical alterations and metal decorporation in rats. Chem Biol Interact 1990; 75(3):341-7. [ Links ]
60. Rubanyi G, Bakos M, Hajdu K, Pataki T: Dependence of nickel- induced coronary vasoconstriction on the activity of the electrogenic Na+, K+-pump. Acta Physiol Acad Sci Hung 1982; 59(2):169-74. [ Links ]
61. Parcell S: Sulfur in human nutrition and applications in medicine. Altern Med Rev 2002; 7(1):22-44. [ Links ]