Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Nutrición Hospitalaria
versión On-line ISSN 1699-5198versión impresa ISSN 0212-1611
Nutr. Hosp. vol.28 no.1 Madrid ene./feb. 2013
https://dx.doi.org/10.3305/nh.2013.28.1.6165
COMUNICACIÓN BREVE
High-protein diets and renal status in rats
Dietas hiperproteicas y estado renal en ratas
V. A. Aparicio1, E. Nebot1, R. García-del Moral2, M. Machado-Vílchez3, J. M. Porres1, C. Sánchez1 and P. Aranda1
1Department of Physiology, School of Pharmacy, Faculty of Sport Sciences and Institute of Nutrition and Food Technology. University of Granada. Spain.
2Department of Pathologic Anatomy and Institute of Regenerative Biomedicine. School of Medicine. University of Granada. Spain.
3UGC Internal Medicine. Hospital Juan Ramón Jiménez. Huelva. Spain.
This study was supported by the project DEP2008-04376 from the Spanish Ministry of Science and Innovation and grants from the Spanish Ministry of Education (AP2009-5033, AP2009-3173) and Economy and Competitively (BES-2009-013442). The authors want to gratefully Lucía Bustos for her collaboration.
ABSTRACT
Introduction: High-protein (HP) diets might affect renal status. We aimed to examine the effects of a HP diet on plasma, urinary and morphological renal parameters in rats.
Material and methods: Twenty Wistar rats were randomly distributed in 2 experimental groups with HP or normal-protein (NP) diets over 12 weeks.
Results and discussion: Final body weight was a 10% lower in the HP group (p < 0.05) whereas we have not observed differences on food intake, carcass weight and muscle ashes content. No significant clear differences were observed on plasma parameters, whereas urinary citrate was an 88% lower in the HP group (p = 0.001) and urinary pH a 15% more acidic (p < 0.001). Kidney wet mass was ~22 heavier in the HP group (p < 0.001). Renal mesangium area was a 32% higher in the HP group (p < 0.01). Glomerular 1 and 2 were also ~30 higher in the HP diet (p < 0.01 and p < 0.05, respectively) and glomerular area a 13% higher (p < 0.01).
Conclusion: High-protein diet promoted a worse renal profile, especially on urinary and morphological markers, which could increase the risk for developing renal diseases in the long time.
Key words: High-protein diet. Plasma. Urine. Kidney. Renal morphology. Rats.
RESUMEN
Introducción: Las dietas hiperproteicas (HP) pueden afectar la función renal. El objetivo del presente estudio fue examinar los efectos de una dieta HP sobre parámetros renales plasmáticos, urinarios y morfológicos en ratas.
Material y métodos: Veinte ratas Wistar fueron distribuidas aleatoriamente en 2 grupos experimentales con dieta HP o normoproteicas durante 12 semanas.
Resultados y discusión: El peso corporal final fue un 10% inferior en el grupo de dieta HP (p < 0,05) mientras que no se han observado diferencias en la ingesta de comida, peso de la carcasa del animal y el contenido muscular de cenizas. No se observaron claras diferencias en los parámetros plasmáticos, mientras que el citrato urinario fue de un 88% inferior en el grupo de dieta HP (p = 0,001) y el pH urinario un 15% más ácido (p < 0,001). El peso del riñón en sustancia fresca fue un 22% más pesado en el grupo de dieta HP (p < 0,001). El Área mesangial fue un 32% mayor en el grupo HP (p < 0,01). El floculo glomerular 1 y 2 fueron también ~ 30 mayores en la dieta HP (p < 0,01 y p < 0,05, respectivamente) y el área glomerular un 13% mayor (p <0,01).
Conclusión: Una dieta hiperproteica promueve un peor perfil renal, especialmente en los marcadores urinarios y morfológico, que podrían aumentar el riesgo de desarrollar enfermedades renales a largo plazo.
Palabras clave: Dieta hiperproteica. Plasma. Orina. Riñón. Morfología renal. Ratas.
Abbreviations
ANOVA: Analysis of variance.
CKD: Chronic kidney disease.
ER: Endoplasmic reticulum.
GFR: Glomerular filtration rate.
HRT: Hypertrophy resistance training.
LDH: Lactate dehydrogenase.
MCP-1: Monocyte chemoattractant protein-1.
N: Nitrogen.
SEM: Standard error of the mean.
Introduction
In the last few years, the use of high-protein (HP) diets (i.e. "The Dr. Dukan diet") is gaining in popularity among the general population. Indeed, HP diets are increasingly being recommended as one of the management strategies for weight control in overweight and obese individuals1-3. High-protein diets appear to reduce appetite, energy intake, body weight, and fat deposition at the same time that improve plasma lipid profile4-5. In view of the high prevalence of obesity, type 2 diabetes, and metabolic syndrome6, it is important to understand the effects of high levels of protein intake on health. This is particularly important for the kidney, because the above mentioned patients are characterized by renal hyperfiltration and increased risk of kidney disease7-9.
Despite the antiobesity effects of HP diets, the impact of such diets on renal status remains unclear10-12. The potentially harmful effects of dietary proteins on renal function are believed to be due to the 'overwork' induced by such diets on the kidneys. Indeed, HP diets cause elevation of glomerular filtration rate (GFR) and hyperfiltration11. However, some authors affirm that the link between protein-induced renal hypertrophy or hyperfiltration and the initiation of renal disease in healthy individuals has not been clearly demonstrated13. This hyperfiltration could have deleterious consequences in diseased kidneys14, however, in healthy individuals, the impact of consuming HP on renal health is unknown10, 13. Nevertheless, a few studies have observed that the exposure of rodents15-16, cats17 or pigs18 to long-term HP diets results in glomerular hyperfiltration with renal morphologic injuries such as glomerular hypertrophy, and a greater prevalence of renal pathological changes.
The present study aimed to examine the plasma, urinary and morphological renal effects of HP diets in rats.
Materials and methods
Animals and experimental design
A total of 20 young albino male Wistar rats were allocated into two groups (n=10), with HP or normal-protein (NP) diet. The animals, with an initial body weight of 148±6 g, were housed in individual stainless steel metabolism cages designed for the separate collection of urine. The cages were located in a well-ventilated thermostatically controlled room (21±2oC), with relative humidity ranging from 40 to 60%. A 12:12 light-dark (08.00-20.00 h) cycle was implemented. Throughout the experimental period all rats had free access to distilled water and the animals consumed the diet ad libitum. One week prior to the experimental period, the rats were allowed to adapt to the experimental conditions.
Body weight was measured weekly for all animals at the same time and the amount of food consumed by each rat was registered daily.
On week 11, a 12-hour urine sample from each animal was collected for biochemical analysis. Urine volumes were recorded and samples were transferred into graduated centrifuge tubes for the posterior pH, Ca, and citrate analysis.
At the end of the experimental period, the animals were anaesthetized with ketamine-xylacine and sacrificed by cannulation of the abdominal aorta. Blood was collected (with heparin as anticoagulant) and centrifuged at 3000 rpm for 15 minutes to separate plasma that was frozen in liquid N and stored at -80oC. Carcass weight was recorded. Carcass is the weight of the slaughtered animal's cold body after being skinned, bled and eviscerated, and after removal the head, the tail and the feet. Kidneys were extracted, weighed, and immediately the left one was introduced in formalin for the posterior histological analysis.
All experiments were undertaken according to Directional Guides Related to Animal Housing and Care (European Community Council, 1986) 19, and all procedures were approved by the Animal Experimentation Ethics Committee of the University of Granada.
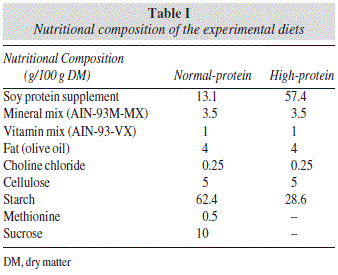
Experimental diet
Formulation of the experimental diet is presented in table I. The diet was formulated to meet the nutrient requirements of adult rats following the recommendations of the American Institute of Nutrition (AIN-93M)20, with slight modifications. We have selected a 45% of protein level for the HP diet group, following previous studies in which HP diet was compared with NP diets in rats4-5,21, whereas a 10% of protein content was chosen for the NP diet group. Commercial soy protein isolate was used as the only source of protein since this protein source is widely available and used by sportsmen. Inclusion of 45% protein level in the diet was done at the expense of complex carbohydrates (wheat starch). Prior to diet preparation, total protein concentration of the commercial isolate was measured. Total N content was 12.4±0.7 g/100g of dry matter, which corresponds to a 77.5% of richness. Total protein concentration of the experimental diet was also assayed, with values of 44.1±2.2% and 9.8±0.4% respectively, for the HP and NP diet.
Chemical analyses
Total N of the soy protein supplement was determined according to Kjeldahl's method. Crude protein was calculated as N x 6.25. Urine Ca content was determined by atomic absorption spectrophotometry using a PerkinElmer Analyst 300 spectrophotometer (PerkinElmer, Wellesley, MA, USA). Analytical results were validated by standard reference materials CRM-189, CRM-383, and CRM-709.
Urinary pH was analyzed with a bench pH-meter (Crison, Barcelona, Spain) and urinary citrate with a commercial kit (Spinreact, S.A. Gerona, España). Plasma total cholesterol, LDL-cholesterol, HDL-cholesterol, triglycerides, urea, total proteins, albumin and lactate dehydrogenase (LDH), were measured with a Hitachi-Roche p800 autoanalyzer.
Histological analysis
Left-kidney samples were fixed in buffered 4% formalin and embedded in paraffin. Afterwards, four-micrometer-thick sections were obtained and stained with 1% Picrosirius red F3BA (Gurr, BDH Chemicales Ltd, Poole, United Kingdom)22. This technique allows the visualization of connective fibers deep red stained on a pale yellow background22. The sections were assessed by optical microscopy. Forty images per sample were captured: twenty of the glomerulus to determine the morphometry and the intraglomerular connective tissue and twenty of the tubulointersticial area to measure the interstitial connective tissue. All images were acquired with 20x objective and analyzed with the Fibrosis HR® software23. This image analysis application allowed us to automatically quantify morphometric parameters by using various image-processing algorithms23.
Statistical analysis
Results are presented as mean and standard error of the mean. Differences between HP and NP diet groups were analyzed by ANOVA; with final body weight, food intake and muscle, urinary, plasma and renal morphology parameters as dependent variables. All analyses were conducted with the Statistical Package for Social Sciences (SPSS, version 19.0 for Windows; SPSS Inc., Chicago, IL), and the level of significance was set at 0.05.
Results
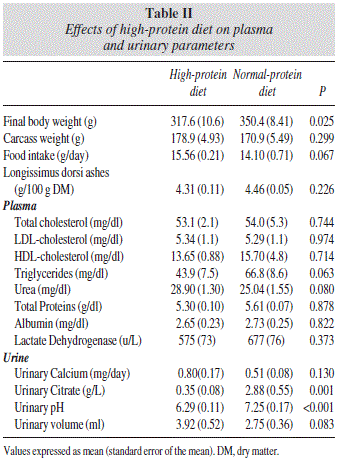
The effects of the HP diet on final body weight, food intake, muscle, plasma and urinary parameters are shown in table II.
Final body weight, food intake and muscle ashes content
Final body weight was a 10% lower in the HP group (p<0.05). No differences were observed in food intake, carcass weight, and muscle ashes content (all, p>0.05).
Plasma and urinary parameters
No significant differences were observed on plasma lipid profile as well as in the rest of renal plasma markers measured (all, p>0.05).
Urinary citrate was an 88% lower in the HP group (p=0.001) and urinary pH a 15% more acidic (p<0.001).
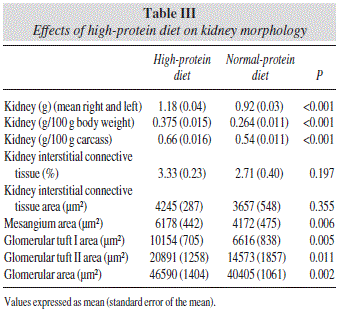
The effects of HP diet on kidney weight and morphology are shown in table III.
Kidney weight and morphology
Kidney wet mass, as expressed in absolute value as well as expressed referred to final body weight or carcass weight, was ~22 higher in the HP group (all, p<0.001). No differences between groups were observed on kidney interstitial connective tissue.
Renal mesangium area was a 32% higher in the HP group (p<0.01). Glomerular tuft 1 and 2 were also ~30 higher in the HP diet (p<0.01 and p<0.05, respectively) and glomerular area a 13% higher in the HP diet (p<0.01).
Discussion
The findings of the present study show: i. HP diet significantly reduced body weight but without clearly improving plasma lipid profile. ii. Urinary citrate and pH were drastically lower in the HP group, which could constitute a favorable environment for kidney stones formation in high-risk patients. iii. The increase of kidney weight observed in the HP groups was accompanied by higher renal mesangiums, glomerular tufts and areas. Therefore, HP diet promoted a worse morphological renal profile.
Under our experimental conditions, the HP diet slightly reduced body weight, which is in agreement with other studies4-5. However, a recent systematic review has observed that the long-term effect of HP diets on weight loss is neither consistent nor conclusive3. Moreover, in contrast to what has been reported by other authors4-5, we have not observed a reduction on food intake and neither a significant better lipid profile. To note is that plasma triglycerides were a 34% lower in the HP diet fed group, which is clinically relevant, but without statistical signification (p=0.063).
Plasma urea concentrations can increase when HP diets are consumed14, 24-25. In the present study, we have observed a close to the signification (p=0.08) 15% higher plasma urea concentrations in the HP diet group. When more urea is excreted, more urea needs to be filtered, and maybe this can be on the basis of the 13% higher glomerular area observed in our groups fed with the HP diet.
Accordingly to the evidence reported previously by our group24,26 and by other studies18, 27-29 we have observed a 22% increase in kidney fresh mass weight of rats after 12 weeks of HP diet consumption. Hammond and James29 found a 26-32% increase in kidney fresh mass weight of rats after 2 weeks of HP diet consumption, which they attributed to the strong effects on blood urea N and totally daily N filtration rate exerted by HP consumption. Some studies performed in rodents27-28 or pigs18 have observed histological damage with HP diets in the long term. In the study by Jia et al.18, whole plant and animal proteins in proportions that mimicked human diets were given to pigs. Adult female pigs received either NP or HP (15 or 35% of energy from protein, respectively) isocaloric diets for either 4 or 8 months. The HP compared with the NP diet resulted in enlarged kidneys at both 4 and 8 months. Renal and glomerular volumes were 60-70% higher by the end of the study. These enlarged kidneys had greater evidence of histological damage, with 55% more fibrosis and 30% more glomerulosclerosis. Similarly, we have observed morphological impairment in our HP diet fed group, with a 32% higher renal mesangium area, a ~30% higher floccules areas and a 13% higher glomerular area in the HP diet groups, but without significant more renal interstitial connective tissue. To note is that we have not observed renal fibrosis.
In the above mentioned study by Jia et al.18, plasma concentrations of homocysteine and renal monocyte chemoattractant protein-1 (MCP-1) were extremely higher in the HP-fed groups18, which could explain the larger kidneys observed. Renal inflammation is induced via release of proinflammatory chemokines, such as MCP-1, which plays an important role in the recruitment of inflammatory cells into the kidney30. Infiltrating inflammatory cells interact with renal cells, causing them to synthesize excessive extracellular matrix, ultimately resulting in the development of kidney interstitial connective tissue30-33.
In contrast, other authors have observed that in long interventional studies performed in humans, including overweight or obese healthy subjects, without preexisting renal dysfunction, the HP diet did not adversely affect renal function, whether it increased GFR and kidney size34 or whether it did not35.
Kidney plays a central role in protein metabolism. Thus, disease states of the kidney invariably result in clinically relevant disturbances of protein metabolism. Conversely, processes regulated by the kidneys are directly affected by dietary protein intake36. The amount and composition of ingested proteins have a direct impact on renal function, especially in a state of diseased kidneys. Consequently, limitation of ingested protein, particularly from animal sources, is crucial in order to slow the progression of chronic kidney disease and impaired renal function36. Relative excess of animal protein ingestion (acid load from sulphur-containing amino acids) might produce intracellular acidosis37. Intracellular acidosis stimulates urinary hypocitraturia, that is often accompanied by urinary hypercalciuria37, which is strongly related to net renal acid excretion38. A decrease in urinary pH, hypocitraturia and hypercalciuria, are recognized risk factors for kidney stone formation, principally by increasing urinary saturation of calcium salts21,37. In our study, the HP diet increased plasma urea and urinary excretion of Ca (close to the statistical signification), at the same time that strongly decreased urinary pH and citrate. Therefore, those animals could be at a higher risk for nephrolithiasis. Furthermore, urine acidification is also a characteristic of visceral obesity and the metabolic syndrome, and thus, HP diets may be associated with various metabolic abnormalities in visceral obesity39.
Something to consider is that the effect of proteins also depends on the presence of other nutrients in the diet. High intakes of fruits and vegetables are associated with a reduced risk for stone formation in high-risk patients40. This beneficial effect of fruits and vegetables is probably due to their high content in potassium and magnesium. Potassium stimulates urinary excretion of citrate, which is an inhibitor of calcium stones formation40-41.
Conclusion
The HP diet consumption promoted, in general, a worse urinary and morphological renal profile, whereas plasma parameters were less clearly affected (showed lower sensitivity to the diet). HP diet significantly reduced body weight but without a parallel improvement on plasma lipid profile. Urinary citrate and pH were drastically reduced by the HP diet, which could constitute a favorable environment for nephrolithiasis in high-risk patients. Finally, the increase of kidney weight, renal mesangiums, glomerular tufts and areas by the HP diet could compromise renal health in the long time.
References
1. Bantle JP, Wylie-Rosett J, Albright AL, Apovian CM, Clark NG, Franz MJ, et al. Nutrition recommendations and interventions for diabetes: a position statement of the American Diabetes Association. Diabetes Care 2008; 31 Suppl 1: S61-78. [ Links ]
2. Lau DC. Synopsis of the 2006 Canadian clinical practice guidelines on the management and prevention of obesity in adults and children. CMAJ 2007; 176: 1103-6. [ Links ]
3. Lepe M, Bacardi Gascon M, Jimenez Cruz A. Long-term efficacy of high-protein diets: a systematic review. Nutr Hosp 2011; 26: 1256-9. [ Links ]
4. Lacroix M, Gaudichon C, Martin A, Morens C, Mathe V, Tome D, et al. A long-term high-protein diet markedly reduces adipose tissue without major side effects in Wistar male rats. Am J Physiol Regul Integr Comp Physiol 2004; 287: R934-42. [ Links ]
5. Pichon L, Potier M, Tome D, Mikogami T, Laplaize B, Martin-Rouas C, et al. High-protein diets containing different milk protein fractions differently influence energy intake and adiposity in the rat. Br J Nutr 2008; 99: 739-48. [ Links ]
6. Shamseddeen H, Getty JZ, Hamdallah IN, Ali MR. Epidemiology and economic impact of obesity and type 2 diabetes. Surg Clin North Am 2011; 91: 1163-72, vii. [ Links ]
7. Agrawal V, Shah A, Rice C, Franklin BA, McCullough PA. Impact of treating the metabolic syndrome on chronic kidney disease. Nat Rev Nephrol 2009; 5: 520-8. [ Links ]
8. Radbill B, Murphy B, LeRoith D. Rationale and strategies for early detection and management of diabetic kidney disease. Mayo Clin Proc 2008; 83: 1373-81. [ Links ]
9. Manabe I. Chronic inflammation links cardiovascular, metabolic and renal diseases. Circ J 2011; 75: 2739-48. [ Links ]
10. Martin WF, Armstrong LE, Rodriguez NR. Dietary protein intake and renal function. Nutr Metab (Lond) 2005; 2: 25. [ Links ]
11. Frank H, Graf J, Amann-Gassner U, Bratke R, Daniel H, Heemann U, et al. Effect of short-term high-protein compared with normal-protein diets on renal hemodynamics and associated variables in healthy young men. Am J Clin Nutr 2009; 90: 1509-16. [ Links ]
12. Friedman AN. High-protein diets: potential effects on the kidney in renal health and disease. Am J Kidney Dis 2004; 44: 950-62. [ Links ]
13. Calvez J, Poupin N, Chesneau C, Lassale C, Tome D. Protein intake, calcium balance and health consequences. Eur J Clin Nutr 2011. [ Links ]
14. Bankir L, Bouby N, Trinh-Trang-Tan MM, Ahloulay M, Promeneur D. Direct and indirect cost of urea excretion. Kidney Int 1996; 49: 1598-607. [ Links ]
15. Bertani T, Zoja C, Abbate M, Rossini M, Remuzzi G. Agerelated nephropathy and proteinuria in rats with intact kidneys exposed to diets with different protein content. Lab Invest 1989; 60: 196-204. [ Links ]
16. Hostetter TH, Meyer TW, Rennke HG, Brenner BM. Chronic effects of dietary protein in the rat with intact and reduced renal mass. Kidney Int 1986; 30: 509-17. [ Links ]
17. Adams LG, Polzin DJ, Osborne CA, O'Brien TD, Hostetter TH. Influence of dietary protein/calorie intake on renal morphology and function in cats with 5/6 nephrectomy. Lab Invest 1994; 70: 347-57. [ Links ]
18. Jia Y, Hwang SY, House JD, Ogborn MR, Weiler HA, OK, et al. Long-term high intake of whole proteins results in renal damage in pigs. J Nutr 2010; 140: 1646-52. [ Links ]
19. Estoppey-Stojanovski L. Position of the Council of Europe on the protection of animals. Dev Biol Stand 1986; 64: 3-5. [ Links ]
20. Reeves PG, Nielsen FH, Fahey GC, Jr. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr 1993; 123: 1939-51. [ Links ]
21. Amanzadeh J, Gitomer WL, Zerwekh JE, Preisig PA, Moe OW, Pak CY, et al. Effect of high protein diet on stone-forming propensity and bone loss in rats. Kidney Int 2003; 64: 2142-9. [ Links ]
22. Sweat F, Puchtler H, Rosenthal SI. Siriu red F3BA as a stain for connective tissue. Arch Pathol 1964; 78:69-72. [ Links ]
23. Masseroli M, O'Valle F, Andujar M, Ramirez C, Gomez-Morales M, de Dios Luna J, et al. Design and validation of a new image analysis method for automatic quantification of interstitial fibrosis and glomerular morphometry. Lab Invest 1998; 78: 511-22. [ Links ]
24. Aparicio VA, Nebot E, Porres JM, Ortega FB, Heredia JM, Lopez-Jurado M, et al. Effects of high-whey-protein intake and resistance training on renal, bone and metabolic parameters in rats. Br J Nutr 2010: 1-10. [ Links ]
25. Frank H, Graf J, Amann-Gassner U, Bratke R, Daniel H, Heemann U, et al. Effect of short-term high-protein compared with normal-protein diets on renal hemodynamics and associated variables in healthy young men. Am J Clin Nutr 2009. [ Links ]
26. Aparicio VA, Nebot E, Kapravelou G, Sanchez C, Porres JM, Lopez Jurado M, et al. Resistance training reduces the metabolic acidosis and hepatic and renal hypertrophy caused by the consumption of a high protein diet in rats. Nutr Hosp 2011; 26: 1478-86. [ Links ]
27. Goldstein DL, Plaga K. Effect of short-term vs. long-term elevation of dietary protein intake on responsiveness of rat thick ascending limbs to peptide hormones. Comp Biochem Physiol A Mol Integr Physiol 2002; 133: 359-66. [ Links ]
28. Bouby N, Trinh-Trang-Tan MM, Laouari D, Kleinknecht C, Grunfeld JP, Kriz W, et al. Role of the urinary concentrating process in the renal effects of high protein intake. Kidney Int 1988; 34: 4-12. [ Links ]
29. Hammond KA, Janes DN. The effects of increased protein intake on kidney size and function. J Exp Biol 1998; 201: 2081-90. [ Links ]
30. El Nahas M. Renal remodelling: complex interactions between renal and extra-renal cells. Pediatr Nephrol 2006; 21: 1637-9. [ Links ]
31. Burt D, Salvidio G, Tarabra E, Barutta F, Pinach S, Dentelli P, et al. The monocyte chemoattractant protein-1/cognate CC chemokine receptor 2 system affects cell motility in cultured human podocytes. Am J Pathol 2007; 171: 1789-99. [ Links ]
32. Giunti S, Tesch GH, Pinach S, Burt DJ, Cooper ME, Cavallo-Perin P, et al. Monocyte chemoattractant protein-1 has prosclerotic effects both in a mouse model of experimental diabetes and in vitro in human mesangial cells. Diabetologia 2008; 51: 198-207. [ Links ]
33. Yi F, Li PL. Mechanisms of homocysteine-induced glomerular injury and sclerosis. Am J Nephrol 2008; 28: 254-64. [ Links ]
34. Skov AR, Toubro S, Bulow J, Krabbe K, Parving HH, Astrup A. Changes in renal function during weight loss induced by high vs low-protein low-fat diets in overweight subjects. Int J Obes Relat Metab Disord 1999; 23: 1170-7. [ Links ]
35. Brinkworth GD, Buckley JD, Noakes M, Clifton PM. Renal function following long-term weight loss in individuals with abdominal obesity on a very-low-carbohydrate diet vs high-carbohydrate diet. J Am Diet Assoc 2010; 110: 633-8. [ Links ]
36. Ambuhl PM. Protein intake in renal and hepatic disease. Int J Vitam Nutr Res 2011; 81: 162-72. [ Links ]
37. Pak CY. Pharmacotherapy of kidney stones. Expert Opin Pharmacother 2008; 9: 1509-18. [ Links ]
38. Tylavsky FA, Spence LA, Harkness L. The importance of calcium, potassium, and acid-base homeostasis in bone health and osteoporosis prevention. J Nutr 2008; 138: 164S-5S. [ Links ]
39. Otsuki M, Kitamura T, Goya K, Saito H, Mukai M, Kasayama S, et al. Association of urine acidification with visceral obesity and the metabolic syndrome. Endocr J 2011; 58: 363-7. [ Links ]
40. Frassetto L, Kohlstadt I. Treatment and prevention of kidney stones: an update. Am Fam Physician 2011; 84: 1234-42. [ Links ]
41. Demigne C, Sabboh H, Remesy C, Meneton P. Protective effects of high dietary potassium: nutritional and metabolic aspects. J Nutr 2004; 134: 2903-6. [ Links ]
![]() Correspondence:
Correspondence:
Virginia A. Aparicio García-Molina.
Department of Physiology.
School of Pharmacy.
University of Granada.
Campus Universitario de Cartuja, s/n.
18071 Granada (Spain).
E-mail: virginiaparicio@ugr.es
Recibido: 11-IX-2012.
Aceptado: 10-X-2012.