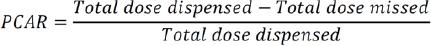INTRODUCTION
Diabetes is looming as one the most serious global epidemic in the 21st century. It is associated with significant morbidity and mortality.1 In Malaysia, the prevalence of diabetes among adults increased from 11.2% (i.e. an estimated 1.9 million in 2011) to 13.4% in 2015 (i.e. an estimated population of 2.7 million) and 18.3% in 2019 (i.e. an estimated population of 3.9 million).2 By comparison, the prevalence of diabetes among adults in Malaysia is much higher than the average prevalence in the South-East Asia region (8.4%) based on a survey in 2014.3 In view of the worsening epidemic, the Ministry of Health (MOH) has undertaken various preventive and awareness programmes such as health campaigns, home medication review (HMR) by pharmacists, and medication therapy adherence clinic for diabetes patients. Despite these efforts, the prevalence continues to rise.
HMR, also known as pharmacist-led medication review, is defined as “a systematic assessment of consumer's medication and management of those medications to optimise health outcome of the consumer and to identify any potential medication-related issues within the framework of the quality use of medicine”.4 In many developed countries such as Australia, New Zealand, the United Kingdom (UK), and the United States of America (US), the models of pharmacist-led medication review in the community or residential aged care setting are very well established.4 Numerous studies have demonstrated that HMR could benefit patients with multiple chronic conditions, increasing age, or adverse social circumstances. The benefits were also observed among patients with complex drug regimen and those with a lack of knowledge or skill on using medications or medication-related devices.5,6
In Malaysia, HMR was initiated by the Pharmaceutical Services Division, Ministry of Health (MOH). In 2011, the first protocol for HMR was published to standardise the practice of HMR services by all the pharmacists under MOH facilities.7 Subsequently, in 2019, MOH published the second version of HMR, known as Home Care Pharmacy Services (HCPS).8 HCPS is one of the pharmaceutical care programmes to ensure the continuity of care upon returning from health facilities i.e. outpatient or inpatient settings to their homes in order to facilitate efficient and quality use of medicines. The entire procedure of HCPS involves extensive and systematic activities that include drug reconciliation and medication analysis for both prescription and non-prescription drugs. MOH pharmacists from hospitals or health clinics are in charge of conducting the HMR or HCPS. The service focuses primarily on four types of patients, namely psychiatry, geriatrics, stroke, and paediatrics. All the HMR or HCPS activities are fully funded by the government. To date, only two studies regarding HMR services have been conducted in Malaysia. Both involved services provided by the MOH pharmacists to T2DM patients in MOH health clinics. The findings revealed that HMR significantly reduced the percentage of HbA1C by 1.04%, apart from improving patients' knowledge about the disease and quality of life (QoL).9,10
Globally, there are very few published studies that evaluated the HMR services provided by community pharmacists.11 Likewise, little is known about the potential role of community pharmacists in providing such services in Malaysia. The community pharmacists are one of the frontliners in the primary healthcare setting as they are responsible to ensure that medication use by the patients is safe, effective, and efficient.12,13 In Malaysia, the number of community pharmacists has significantly increased from 1,854 in the year of 2011 to 3,094 in the year 2016.14 With the increasing manpower, the evolution in the role of community pharmacists in pharmaceutical care provision is therefore anticipated, and HMR could be one of it. HMR is a comprehensive clinical review of a patient's medicines at their homes by accredited community pharmacists (HMR-CP) upon referral from the family medicine specialist (FMS), medical officers (MO), or general practitioner (GP). This service is believed to be particularly beneficial for T2DM patients who live independently in the community.15 However, further investigations are warranted to establish this association. This randomised clinical trial (RCT) aimed to evaluate the effectiveness of the HMR-CP programme in optimising diabetes care in Malaysia. The clinical impact of HMR-CP (i.e. glycaemic control, blood pressure, anthropometric parameters, lipid profiles), number of DRP resolved or prevented, rate of severe adverse events, hospitalisation, and humanistic outcomes (i.e. QoL, patients' knowledge about the disease, and medication adherence) among patients with T2DM were evaluated.
METHODS
Ethics approval and trial registration
The study was approved by the Medical Research & Ethics Committee (MREC), Ministry of Health (MOH) [Reference number: NMRR-17-2348-37624(IIR)], and the UiTM Research Ethics Committee (Reference number: REC/224/18). This trial was also registered with the Australian-New Zealand Clinical Trial Registry (ANZCTR) (ACTRN12618000839202).
Study design and setting
An RCT was conducted between March to December 2018. T2DM patients were recruited from the Bandar Pasir Mas Healthcare Clinic in Kelantan, Malaysia. This clinic is one of the public primary healthcare clinics in the state of Kelantan, serving a total of 189,292 outpatients in the surrounding areas.16 Kelantan is located in the North-Eastern region of Peninsular Malaysia. In 2012, the state of Kelantan recorded the lowest rate of diabetes patients (14.9%) with a satisfactory HbA1c level of ≤ 6.5% among all the states in Malaysia.17 Thus, it was purposively chosen as the site of this trial.
Study population
The inclusion criteria for this RCT were (i) adult patients age 18 years and above, (ii) latest HbA1c of more than 6.5%, (iii) took five or more medications for long-term maintenance therapy or taking more than 12 doses of medications daily, (iv) stayed within 25 km radius from the Bandar Pasir Mas Health Clinic and (v) can be contacted through phone. These inclusion criteria were based on the MOH guideline that specified the requirements for patients to be included in HMR.7
Patients were excluded if they endured mental health problems, were dependant on their caretakers, unable to communicate in English or Bahasa Malaysia, had no permanent residential address, with blood disorders (i.e. haemolytic anaemia, haemoglobinopathy, chronic lymphocytic leukaemia), or diagnosed with Stage 3 to 5 of chronic kidney disease.18,19 A detailed explanatory statement was given to them and informed consent was signed by all participants prior to the commencement of the study. The participants were randomly assigned to an intervention group (i.e. receiving HMR-CP) or a control group (i.e. not receiving HMR-CP). All participants were followed up for six months.
Sample size calculation
The primary outcome of this study was the change in HbA1c between HMR-CP and control groups. A reduction of 1% HbA1c was associated with a 21% risk reduction of death related to diabetes, 37% risk reduction for microvascular complications, 21% risk reduction of diabetes related-end point, and 14% risk reduction of myocardial infarction.20 Hence, a 1% reduction in HbA1c was considered to be clinically relevant. Based on an expected mean difference of HbA1c of 1.04% between the groups, standard deviation of 2.16%, power of 80%, and a two-tailed alpha of 0.05, the required sample size was calculated to be at least 69 intervention subjects and 69 control subjects.10 Taking into account a dropout/loss to follow-up rate of 20%, at least 166 participants were required (i.e. 83 experimental subjects and 83 control subjects). The sample size was calculated using PS Power and Sample Size Calculations (Version 3.0, Dupont & Plummer, 2009).
Intervention
The HMR protocol established by the Pharmaceutical Services Division, MOH was used as the reference to develop the forms and materials for the HMR-CP programme.7 Pilot testing was conducted using all the materials to determine the feasibility of the HMR-CP programme in the community setting and to assess the comprehension level of the FMS/MO and the community pharmacists towards the developed programme materials. Any feedback and comments were taken into consideration. All language discrepancies and grammar mistakes were rectified before the materials for the HMR-CP programme were finalised.
A toolkit that comprised a set of standardised forms was prepared. It was provided to all the community pharmacists who were involved in this programme to ensure that consistent information was delivered to all study participants. The community pharmacists in Kelantan were invited to join this programme through the Malaysian Community Pharmacy Guild (MCPG) Kelantan branch. To enlist the help of MCPG, a letter was sent to the President of the Malaysian Community Pharmacy Guild (MCPG) and a presentation of this project was given at one of MCPG's regular seminars. A total of seven community pharmacists who fulfilled the inclusion criteria (i.e. hold a valid Licence A) and agreed to participate in the HMR-CP programme were recruited and trained. All FMS/MO involved in diabetes care at the Bandar Pasir Mas Health Clinic participated in this study. A one-day training session was conducted by the researchers to train the community pharmacists and other FMS/MO on how to conduct HMR-CP, fill-up the forms, and write the report. The community pharmacists were also briefed on the type of medication available at the Bandar Pasir Mas Health Clinic and the routine management of T2DM by the FMS.
The study intervention (i.e. HMR-CP) involved both HMR visits performed by community pharmacists and review by FMS/MO. The community pharmacists visited T2DM participants' houses every three months (i.e. baseline, 3-month, and 6-month). The three time-points (i.e. 0-, 3-, and 6-month) were selected with the aims of determining the short-term and long-term impacts of the HMR-CP programme on both clinical and humanistic outcome measures.
Within one week of obtaining the patients' consent, the researcher contacted those who were assigned to the HMR-CP group to arrange an appointment for the community pharmacists to make the first visit to their homes. Community pharmacists were provided with patient profiles, medication history, clinical, and anthropometric data for the counselling session. Each HMR visit lasted between 20 and 45 minutes in which the community pharmacists provided tailored counselling to the participants about medication adherence, lifestyle modification, and self-monitoring blood glucose monitoring at home. The counselling on medication adherence involved emphasising the importance of adhering to prescribed medication, calculating the balance of pills to determine the adherence, assessing patient's long-term and short term goals, and creating awareness on the common side effects of the medication, as well as reminding them of the consequences and action to be taken in case of a missed dose. For lifestyle modification, community pharmacists discussed nutritional intake in terms of the types of food, quantity per serving, and timing of meals. Patients were also counselled on suitable physical activities according to their current health conditions, the importance of weight management, and glucose monitoring. Any hospital admission was also collected and recorded. After each HMR visit, the community pharmacists completed a report to provide feedback (including any detected drug-related problems) to the FMS/MO so that adjustments in patients' medication regimens could be made where necessary.
Participants who were randomised to the control group were provided with usual care without any active intervention. They received routine treatment by MOs in the health clinic and were contacted through phone by the researcher to remind them about their visits to the health clinic for blood sampling and data collection. The researcher interviewed them through the phone to obtain their responses for the MDKT and EQ-5D-5L questionnaires. All the responds were recorded in hardcopy of MDKT and EQ-5D-5L questionnaires.
Primary outcome
For diabetes, HbA1c is a clinically relevant parameter and thus the preferred test for assessing glycaemic control in people with diabetes. The value of 6.5% is recommended as the cut-off point for the diagnosis of diabetes.19,21,22 The baseline HbA1c value was retrieved from the patients' medical records (e.g. not more than three months). About 5 mL of venous blood was withdrawn by an appointed nurse or MO during the follow-up at 3-month and 6-month for both groups. Blood samples were sent to the laboratory in the Bandar Pasir Mas Healthcare Clinic and the HbA1c levels were documented by investigators using a standardised, investigator-developed data collection form.
Secondary outcomes
Clinical and anthropometric parameters: In this study, secondary outcomes included fasting blood glucose (FBG), and diastolic blood pressure (DBP) were obtained at baseline, 3-month, and 6-month follow-up. Similarly, weight, height, and body mass index (BMI) were measured and documented using a standardised, investigator-developed data collection form at similar intervals. All the secondary outcome measures for both groups were recorded by an appointed nurse at baseline, 3-month, and 6-month follow-up.
Medication-related issues and hospitalisation: In this study, DRP was only recorded for the intervention group during each HMR-CP visit. Classification for DRP(Version 8.02) developed by the Pharmaceutical Care Network Europe Foundation (PCNE) was used with permission to classify the drug-related problems.23 Twenty percent of patients in the intervention group were randomly chosen and the classification of their DRP was discussed within a multidisciplinary team of FMS/MO, community pharmacists, and researchers. Any discrepancy was resolved with team consensus. The PCNE code for the remaining 80% of patients was determined by the researchers. In addition, hospitalisation or visits to the emergency department due to adverse events of anti-diabetic medications or hypoglycaemic attacks during the six months of the study were recorded. For the HMR-CP group, patients were asked about hospital admission by the community pharmacist during home visits. As for the control group, the information on hospital admission was obtained via follow-up phone calls.
Medication adherence: It is well-known that treatment failure due to poor medication adherence can result in many re-hospitalisations, poor disease outcomes, and increased healthcare costs.24 In this trial, medication adherence for the intervention group was determined based on pill counting adherence ratio (PCAR) and subsequently documented in the HMR-CP form. During each HMR-CP visit, the community pharmacist calculated the remaining pills of the T2DM patients to check if the balance tallied it with what they should have based on the prescribing record. In the Malaysian health clinic setting, each patient is supplied with medication by the pharmacy department monthly. Before dispensing, the pharmacist at the health clinic will ask the patient about the balance of medication before supplying them with medication that is sufficient for one month.
The formula above was used to calculate the adherence ratio.25 The higher the ratio, the higher the patient's adherence rate towards medication. The cost of medication wastage was then calculated based on the total dose missed by the T2DM patients multiplied by cost of medication paid by the MOH.
Study instruments
The permission to use the five-level EuroQol-5 Dimension Questionnaire (EQ-5D-5L) and the Malaysian Version of the Michigan Diabetes Knowledge Test (MDKT) were obtained from the respective authors or organisations before the commencement of the study. As a recent study reported that no significant change was observed in the QoL of patients within a short intervention period, the QoL and participants' knowledge were only assessed at baseline and 6-month follow-up.26 For those in the HMR-CP group, these data were collected during the HMR-CP home visits while participants in the control group provided these data via phone calls. The difference in the data collection method between the two groups arose since the community pharmacists did not have any direct contact with patients in the control group. Therefore, it was more feasible for the researcher to collect these data via phone calls. Despite the difference, the tools used were the same for both groups.
The general health of the participants was assessed using the reliable and validated EQ-5D-5L questionnaire.27 The descriptive index obtained was converted to a utility value. EQ-5D-5L is a descriptive system of health-related QoL states that consists of five dimensions (i.e. mobility, self-care, usual activities, pain/discomfort, and anxiety/depression), each of which can be responded in five levels of severity (i.e. no problem, slight problem, moderate problem, severe problem, extreme problem). The validated English and Malay-translated versions of EQ-5D-5L from EuroQoL was used and the recently published Malaysian EQ-5D-5L value set was applied for the health state valuation in this RCT.28
The patient's self-management is regarded as one of the critical factors to ensure good control of blood glucose level and high QoL among diabetic patients. To achieve good self-disease management, diabetic patients must possess sufficient knowledge and skills for their daily care management.29 In this RCT, the participant's knowledge about diabetes was measured using the Malaysian version of 14-item Michigan Diabetes Knowledge Test (MDKT). This instrument has been proven to be a reliable and valid measure of diabetes knowledge that can be used in both clinical and research practice.30
Randomisation procedure, concealment of allocation, and blinding
A set of random numbers was generated using Microsoft Excel and was kept in a sealed envelope by an independent researcher. Blinding refers to the concealment of group allocation from one or more individuals who are involved in an RCT.31 In this single-blinded RCT, the appointed nurse who took the blood samples for clinical outcomes (i.e. glycaemic parameters, blood pressure, and lipid profiles), and also anthropometric data [i.e. weight, body mass index (BMI)] was blinded from knowing the group of patients.
Statistical analysis
All data were analysed using statistical software IBM SPSS version 24 (IBM Corp, Chicago, Illinois, USA). Non-normally distributed data were log-transformed into a normalised distribution before analysis. Intention-to-treat (ITT) population analysis was performed on a complete set of data after the missing data were imputed using the last observation carried forward (LOCF) method. Categorical variables were presented using descriptive statistics i.e. frequency and percentage whereas continuous data were presented as mean (standard deviation). Chi-square was used for the comparisons of categorical data. The independent t-test was used to compare the baseline parameters between both groups. In addition, repeated measures analysis using General Linear Model (GLM) was used to compare data across the different time-points within the group. The difference in changes from baseline to 6-month HbA1c between the control and intervention groups was analysed using the Generalised Estimating Equations (GEE) to determine the correlation of measurements at different time points for each individual. In the regression model, age, gender, ethnicity, BMI, FBG, intervention, and time were considered as covariates or factors. FBG was included in the model for adjustment as it was not comparable between the two groups at the baseline. The same linear model was also applied to all the secondary outcomes and the clinical parameters that were used to simulate the lifetime health outcome. Models with intervention x time interaction effects were also examined to determine how effectiveness changed over time according to the intervention assignment. A priori level of significance of 0.05 was set for the study.
RESULTS
Of the 3,110 patients screened for eligibility using the National Diabetic Registry at the Bandar Pasir Mas Health Clinic, 1,244 of them fulfilled the inclusion criteria and were approached by the researchers. A total of 166 patients with T2DM agreed to participate and were then randomly assigned to either HMR-CP or control groups (Figure 1). However, 17 of them [HMR-CP group, n=9 (10.8%); control group, n=8 (9.6%), p=0.798] dropped out of the study at the 6-month follow-up, thus giving rise to an attrition rate of 10.2%. The demographic characteristics [i.e. age (p=0.340), gender (p=0.589), and ethnicity (p=0.155)] and the clinical profiles [i.e. use of insulin (p=0.202), BMI (p=0.558), HbA1c (p=0.060), FBG (p=0.045), and DBP (p=0.690)] were similar between the dropouts and the participants who remained in the study.
The mean age of the 166 participants was 62.0 years old (SD 7.93). The majority were females (75.3%) and Malays (98.8%). The mean HbA1c level and mean BMI were 9.91% (SD 2.16) and 27.79 kg/m2 (SD 5.02), respectively. Table 1 shows the demographic characteristics for both groups. There was no significant difference between both groups at baseline, except for the proportion of baseline FBG (p=0.045). The participants assigned to the control group had a lower baseline FBG (9.5 mmol/L, SD 3.81) compared with the intervention group (10.9 mmol/L SD 4.56). In both groups, the majority of the participants were females and of Malay ethnicity. The mean BMI for each group was 28 kg/m2, indicating most of the participants were either overweight or obese. Almost all participants were prescribed with statin [HMR-CP group, n=81 (97.6%); control group, n=83 (100.0%), p=0.155]. Apart from that, no other significant differences were observed between the two groups at baseline for all the above mentioned outcome measures (i.e. glycaemic parameters, anthropometric parameters, blood pressure, lipid profiles, QoL, and MDKT score).
Table 1. Demographic data of the participants in each group at baseline
| Characteristics | Control group (n=83) | HMR-CP group (n=83) | p-value |
|---|---|---|---|
| Age (years), mean (SD) | 61.36 (8.50) | 62.54 (7.36) | 0.340a |
| Gender, n (%) | 0.589b | ||
| Male | 22 (26.5) | 19 (22.9) | |
| Female | 61 (73.5) | 64 (77.1) | |
| Ethnicity, n (%) | 0.155b | ||
| Malay | 81 (97.6) | 83 (100.0) | |
| Chinese | 2 (2.4) | 0 (0.0) | |
| Anthropometric data, mean (SD) | |||
| Height (cm) | 155.58 (7.29) | 156.04 (7.81) | 0.697a |
| Weight (kg) | 66.84 (13.83) | 68.46 (14.60) | 0.464a |
| BMI (kg/m2) | 27.56 (5.06) | 28.02 (5.01) | 0.558a |
| Clinical parameters, mean (SD) | |||
| HbA1c (%) | 9.60 (2.21) | 10.23 (2.08) | 0.060a |
| FBG (mmol/L) | 9.54 (3.81) | 10.93 (4.56) | 0.045a,c |
| DBP (mmHg) | 75.69 (10.21) | 76.30 (9.61) | 0.690a |
| Anti-diabetic medicine, n (%) | |||
| Metformin | 76 (91.6) | 70 (84.3) | 0.153b |
| Gliclazide | 42 (50.6) | 36 (43.4) | 0.351b |
| Glibenclamide | 3 (3.6) | 2 (2.4) | 0.650b |
| Acarbose | 4 (4.8) | 2 (2.4) | 0.406b |
| Insulin | 47 (56.6) | 55 (66.3) | 0.202b |
| Anti-hypertensive medicine | 82 (98.8) | 83 (100.0) | 0.316b |
| Cholesterol-lowering medicine | 83 (100.0) | 81 (97.6) | 0.155b |
| Respiratory medicined | 5 (6.0) | 2 (2.4) | 0.247b |
| Other type(s) of medicinee | 76 (91.6) | 70 (84.3) | 0.153b |
| Medication cost per month, mean (SD) | |||
| Total medicine cost (MYR) | 59.59 (46.78) | 65.48 (39.90) | 0.384a |
| Anti-diabetic medicine cost (MYR) | 35.18 (40.29) | 40.71 (28.32) | 0.308a |
| Other medicine cost (MYR) | 48.68 (220.31) | 25.60 (27.53) | 0.345a |
aIndependent t-test
bChi-square test
cLog-transformed independent t-test
dBronchodilators, Anti-inflammatory agents, and Leukotriene antagonists
eVitamin, Antibiotics and as-needed based medicine
Furthermore, the incidence of hospitalisation was comparable between both groups [HMR-CP group, n=3 (3.6%); control group, n=6 (7.2%), p=0.304]. No serious adverse event was reported for both groups throughout the intervention period apart from one participant in the control group who passed away due to a heart attack.
Table 2 summarises the changes of glycaemic parameters and all the outcome measures across all time-points for the HMR-CP and control groups. Significant reduction in HbA1c from 10.29% (SD 2.08) to 9.32% (SD 2.19) (p<0.001) was noted within the HMR-CP group while no significant change was observed in the control group. As revealed in the GEE analysis, HbA1c decreased significantly over time in the HMR-CP group at 6-month follow-up (beta= -0.386, 95%CI: -0.647 to -0.126, p=0.004) after adjusting for baseline BMI, FBG, and demographic variables. A similar observation was noted for FBG. Significant reduction in the FBG was noted within the HMR-CP group from 10.93 mmol/L (SD 4.56) to 9.31 mmol/L (SD 4.38) (p=0.002). The opposite was observed in the control group whereby FBG increased from 9.54 mmol/L (SD3.81) to 9.85 mmol/L (SD 3.77) (p=0.086). The GEE analysis also showed that FBG significantly decreased over time in the HMR-CP group at 6-month follow-up (beta= -0.038, 95%CI: -0.069 to -0.008, p=0.015) after adjusting for baseline values and demographic variables. For all the outcomes, there were 10.8% of missing values at most. Thus, they were not associated with the main covariables and assumed to be missing at random.
Table 2. Clinical variables and anthropometric data throughout the study
| n | Mean (SD) | Within group p-valuea | 3-month-baseline | 6-month-baseline | Interaction p-valueb | |||
|---|---|---|---|---|---|---|---|---|
| Baseline | 3-month | 6-month | ITT | |||||
| Glycaemic parameters | ||||||||
| HbA1c (%) | 0.004* | |||||||
| HMR-CP group | 83 | 10.29 (2.08) | 10.00 (2.19) | 9.32 (2.16) | <0.001* | -0.22 (1.23) | -0.91 (1.70) | |
| Control group | 83 | 9.60 (2.21) | 9.89 (2.27) | 9.60 (1.88) | 0.134 | 0.29 (1.20) | -0.01 (1.70) | |
| FBG (mmol/L) | 0.015c* | |||||||
| HMR-CP group | 83 | 10.93 (4.56) | 9.60 (3.99) | 9.31 (4.38) | 0.002c* | -1.33 (4.67) | -1.62 (4.80) | |
| Control group | 83 | 9.54 (3.81) | 10.59 (4.59) | 9.85 (3.77) | 0.086c | 1.05 (4.38) | 0.31 (4.26) | |
| Anthropometric measurements | ||||||||
| Weight (kg) | 0.409 | |||||||
| HMR-CP group | 83 | 68.46 (14.60) | 68.08 (14.27) | 68.22 (14.43) | 0.184 | -0.38 (1.67) | -0.24 (2.24) | |
| Control group | 83 | 66.84 (13.83) | 66.32 (13.66) | 66.45 (13.91) | 0.030* | -0.53 (1.68) | -0.39 (2.04) | |
| BMI (kg/m2) | 0.575 | |||||||
| HMR-CP group | 83 | 28.02 (5.01) | 27.87 (4.96) | 27.93 (5.04) | 0.224 | -0.15 (0.67) | -0.09 (0.91) | |
| Control group | 83 | 27.56 (5.06) | 27.35 (5.01) | 27.40 (5.09) | 0.039* | 0.21 (0.71) | -0.16 (0.87) | |
| Diastolic BP (mmHg) | 0.046* | |||||||
| HMR-CP group | 83 | 76.30 (9.61) | 76.52 (8.72) | 76.33 (9.89) | 0.962 | 0.22 (7.38) | 0.02 (9.68) | |
| Control group | 83 | 75.69 (10.21) | 78.46 (10.50) | 78.65 (9.21) | 0.005* | 2.77 (9.83) | 2.96 (9.13) | |
aRepeated measures (generalised linear model)
bGeneralised estimating equation (adjusted for age, gender, ethnicity, BMI, FBG, time, intervention, intervention x time interaction)
cLog-transformed data
Within the control group, there was a significant weight reduction (p=0.030) and BMI (p=0.039) as compared to the HMR-CP group that reported no significant changes in these two parameters. Despite adjusting for the baseline values and demographic variables, changes in other anthropometric parameters such as weight (p=0.409) and BMI (p=0.575) did not differ significantly between the groups over time. On the other hand, DBP increased significantly within the control group (p=0.005) as indicated by the GLM analysis. The GEE analysis showed a significant decrease of DBP over time in the HMR-CP group at 6-month follow-up (beta= -1.459, 95%CI: -2.889 to -0.029, p=0.046).
The change in TC within each group was significant but not for HDL (Table 3). Likewise, the GEE analysis showed a significant decrease of TC over time in the HMR-CP group at 6-month follow-up (beta= -0.315, 95%CI: -0.444 to -0.186, p<0.001) after adjusting for baseline numbers and demographic variables. However, a significant reduction in HDL over time was noted in the HMR-CP group at 6-month follow-up (beta= -0.035, 95%CI: -0.063 to -0.007, p=0.015) after adjusting for baseline BMI, FBG and demographic variables.
Table 3. Pre- and post-intervention data for lipid profile, utility value and diabetes knowledge
| Variables | n | Mean (SD) | Within group p-valuea | Mean difference | Interaction p-valueb | |
|---|---|---|---|---|---|---|
| Pre-intervention | Post-intervention | ITT | ||||
| Knowledge | ||||||
| MDKT total score | ||||||
| HMR-CP group | 74 | 10.77 (2.45) | 13.62 (0.75) | <0.001 | 2.85 (2.23) | <0.001 |
| Control group | 83 | 10.23 (2.01) | 10.99 (1.71) | <0.001 | 0.76 (1.07) | |
| Quality of life | ||||||
| ED-5Q-5L utility value | <0.001 | |||||
| HMR-CP group | 74 | 0.82 (0.16) | 0.90 (0.10) | <0.001 | 0.08 (0.11) | |
| Control group | 83 | 0.82 (0.10) | 0.80 (0.12) | 0.024 | -0.01 (4.59) | |
| Lipid profiles | ||||||
| TC (mmol/L) | <0.001 | |||||
| HMR-CP group | 74 | 5.69 (1.43) | 5.39 (1.22) | 0.002 | -0.34 (0.89) | |
| Control group | 73 | 5.46 (1.10) | 5.74 (1.12) | <0.001 | 0.28 (0.65) | |
| HDL (mmol/L) | 0.015 | |||||
| HMR-CP group | 74 | 1.28 (0.32) | 1.25 (0.31) | 0.163 | -0.03 (1.91) | |
| Control group | 73 | 1.26 (0.31) | 1.29 (0.31) | 0.052 | 0.03 (0.15) | |
aRepeated measures (generalised linear model)
bGeneralised estimating equation (adjusted for age, gender, ethnicity, BMI, FBG, time, intervention, intervention x time interaction)
As shown in Table 3, both the utility value and MDKT score increased significantly within the HMR-CP group. However, for the control group, the utility value decreased significantly while the MDKT score increased significantly. The differences in the utility and MDKT scores pre- and post-intervention were significant between groups (p<0.001). Likewise, the GEE analysis showed a significant increase of utility value over time in the HMR-CP group at 6-month follow-up (beta= 0.049, 95%CI: 0.036 to 0.063, p<0.001) after adjusting for baseline values and demographic variables. A similar observation was noted in the knowledge score (Table 3). MDKT score increased significantly over time in the HMR-CP group at 6-month follow-up (beta= 1.040, 95% CI: 0.760 to 1.319, p<0.001) after adjusting for baseline BMI, FBG, and demographic variables.
Table 4 outlines the characteristics of DRP based on the PCNE classification. The five components of PCNE included the problem, cause of the problem, planned intervention, acceptance of intervention proposed, and outcome of the intervention. The frequency of DRP reduced from 68 to 43 (p<0.001) and the causes of the problem also declined from 150 to 72 (p<0.001) over time in the HMR-CP group. Upon the completion of the first HMR-CP session, the most commonly planned interventions were drug counselling for patients (n=66, 48.18%) and informing the prescriber about the findings (n=65, 47.45%) based on the HMR-CP report, for example, whether the patient uses/takes less drug than prescribed or does not take the drug at all, inappropriate storage of drugs, inappropriate timing of consumption or dosing intervals, etc. Next, most of the proposed intervention were accepted, either with full (n=22, 31.88%) or partial (n=30, 43.48%) implementation. Likewise, the majority of the identified DRP were either totally (n=23, 33.82%) or partially (n=28, 41.18%) solved.
Table 4. Characteristics of drug-related problems using PCNE classification
| Variables | Number | p valuea | ||
|---|---|---|---|---|
| Baseline | 3-month | 6-month | ||
| Number of drug-related problems | <0.001 | |||
| P1.2 An effect of the drug treatment not optimal | 67 | 51 | 42 | |
| P2.1 Adverse drug event (possibly) occurring | 1 | 2 | 1 | |
| Number of causes for drug-related problem | <0.001 | |||
| C1.1 Inappropriate drug according to guidelines/ formulary | 2 | 1 | 0 | |
| C1.3 No indication for drug | 1 | 0 | 0 | |
| C2.1 Inappropriate drug form (for this patient) | 2 | 3 | 2 | |
| C3.2 Drug dose to high | 0 | 0 | 1 | |
| C4.2 Duration of treatment to long | 0 | 1 | 0 | |
| C7.1 Patients uses/takes less drug than prescribed or does not take the drug at all | 52 | 45 | 35 | |
| C7.2 Patients uses/takes more drug than prescribe | 0 | 0 | 1 | |
| C7.3 Patients abuses drug (unregulated overuse) | 3 | 1 | 0 | |
| C7.4 Patients uses unnecessary drug | 1 | 0 | 0 | |
| C.7.6 Patient store drug inappropriately | 34 | 19 | 8 | |
| C7.7 Inappropriately timing or dosing intervals | 49 | 33 | 23 | |
| C7.8 Patient administers/uses the drug in a wrong way | 3 | 0 | 0 | |
| C7.9 Patient unable to use drug/form as directed | 3 | 2 | 2 | |
| Planned of intervention (prescriber) | <0.001 | |||
| I1.1 Prescriber informed only | 65 | 52 | 41 | |
| I1.3 Intervention proposed to prescriber | 4 | 2 | 1 | |
| Planned of intervention (patients) | 0.002 | |||
| I2.1 Patients (drug) counselling | 66 | 51 | 41 | |
| I2.4 Spoken to family members/caregiver | 2 | 2 | 2 | |
| Implementation | <0.001 | |||
| A1.1 Accepted and fully implemented | 22 | 14 | 0 | |
| A1.2 Accepted and partially implemented | 30 | 17 | 0 | |
| A1.3 Accepted but not implemented | 16 | 18 | 0 | |
| A1.4 Accepted, implementation unknown | 0 | 0 | 37 | |
| A2.1 Not accepted, not feasible | 0 | 1 | 0 | |
| A2.4 Not accepted, unknown reason | 1 | 2 | 0 | |
| A3.1 Proposed but accepted unknown | 0 | 0 | 3 | |
| Outcome of intervention | <0.001 | |||
| O1.1 Problem totally solved | 23 | 15 | 0 | |
| O2.1 Problem partially solved | 28 | 17 | 0 | |
| O3.1 Problem not solved, lacked cooperation of patients | 16 | 17 | 0 | |
| O3.2 Problem not solved, lacked cooperation of prescriber | 1 | 2 | 0 | |
| O0.1 Problem status unknown | 0 | 0 | 40 | |
aChi-square test
As summarised in Table 5, a significant increase of PCAR from 0.20 (SD 0.21) to 0.51 (SD 0.28) (p<0.001) was noted. There was also a significant reduction in the cost of medication wastage from MYR 21.73 (34.86) to 12.55 (16.41) (p=0.014) among those who received HMR-CP.
Table 5. Changes of medication-related issues within the intervention group throughout the study
| Variables | n | Mean (SD) | p-valuea | ||
|---|---|---|---|---|---|
| Baseline | 3-month | 6-month | |||
| Pill counting adherence ratio (PCAR) | 74 | 0.20 (0.21) | 0.31 (0.25) | 0.51 (0.28) | <0.001 |
| Cost of medication wastage (MYR) | 74 | 21.73 (34.86) | 15.08 (14.98) | 12.55 (16.41) | 0.014 |
aRepeated measures (generalised linear model)
DISCUSSION
To date, there is limited published studies that evaluated medication review services for specific diseases using RCT. In the UK, even after more than a decade of introducing medication-related advance services such as Medicines Use Review (MUR) and New Medicine Service, the impact of these services remains unknown.32,33 A meta-analysis by Aguiar et al. that included 22 RCTs involving pharmacist-led interventions (e.g educating patients, providing medication counselling, sending recommendations to the physician regarding change in medication, adjusting pharmacotherapy, and referring patients to other healthcare professionals) led to significant reduction in the mean HbA1c by 0.85% when compared with the usual care group.34 Similar to another systematic review of community pharmacists' interventions by Chiazor et al., all the interventions in the RCT studies of Aguiar et al. were conducted in the community pharmacy, hospital, or primary care setting. None of the studies were conducted in the setting of the patients' homes.34,35 Additionally, a recent umbrella review by Newman et al. that included nine review papers concluded that CP-led interventions were associated with a significant reduction in HbA1c, total cholesterol, and LDL among the diabetes patients.36 However, all the studies in the nine review papers involved different designs and locations of intervention. Some were face-to-face intervention in the outpatient and inpatient department or during patient's visit to the community pharmacy. Again, none of the studies involved HMR conducted by CP at the patient's home. Another study by Fink et al. (2019) found that T2DM patients who received clinical pharmacist care management of their disease reported a reduced HbA1c by 1.6% compared to the control group with only a 0.9% reduction.37
To the best of our knowledge, this is the first RCT in Malaysia that evaluated the clinical and humanistic outcomes of the HMR program provided by CP at the homes of diabetes patients. A scoping review by Stewart et al. (2019) on CP-led medication review in the UK highlighted that no RCT has been conducted to evaluate the clinical outcomes among diabetic patients. Most of the studies in the scoping review focused on barriers and facilitators of the implementation of medication review, patients' and pharmacists' perception towards the program, the process of conducting the consultation, and patient adherence.33 Furthermore, all the local studies in Malaysia involved only pharmacists from health clinics/hospitals who provided HMR.9,10,38,39 In addition, most of the studies from other countries involved pharmacist-led medication review at healthcare provider settings. Different countries practised different ways of implementing pharmacist-led medication reviews. For example, MUR was used in the UK and New Zealand, whereas the US used Medication Therapy Management (MTM) and Australia practised Home Medication Review (HMR).40 For MUR, the consultation takes place at community pharmacy.41 As for MTM, the medication review is carried out at various healthcare premises.42 Lastly, for HMR, the medication review takes place at the patient's home.43 However, the HMR service in Australia is slightly different from the current HMR-CP project in Malaysia, in which the need for HMR is requested by government MO/FMS. Comparatively, the HMR services in Australia can be ordered by the patient's general practitioner, community pharmacist, accredited pharmacists, patients, and also a caregiver.43 According to the report “Implementing and evaluating a parallel post-discharge Home Medication Review (HMR) model” published by The Pharmacy Guild of Australia, several barriers from both the perspectives of patients and pharmacists might limit the implementation of HMR, such as cultural issues, language differences, and privacy.44 Nevertheless, in this study, all the community pharmacists involved hailed from the same region, thus minimising the potential barriers of cultural and language issues.
In view of the different settings between this RCT whereby community pharmacists performed HMR at patient's homes compared to other published studies, the results cannot be directly compared. Therefore, most of the results were compared with studies that involved health clinic/hospital pharmacists that provided HMR in the Malaysian setting. Generally, our study showed a significant improvement in glycaemic control (i.e. reductions in HbA1c and FBG) in the HMR-CP group compared to the control group. This is in line with other studies that involved pharmacist-led medication review.9,10,38 Based on the positive findings in our study, it is obvious that the job scope of the community pharmacists can also be expanded to the patients' home apart from the usual community pharmacy setting. This can be considered as one of the extended services offered by the CP in the future.
During the first visit of HMR-CP, it was noted that most of the patients took their anti-diabetic medications at an inappropriate time or dosing interval (n=49, 66.2%). Many of them also took medicine of lower dosage than the one prescribed to them or did not take medicine at all (n=54, 70.3%). Slightly less than half of them (n=34, 45.9%) stored their drugs inappropriately, especially insulin. This study also showed that the increase in the knowledge about the disease and better adherence were correlated with a decrease of HbA1c in the intervention group. This finding was in concordance with a previous study in which lower knowledge about diabetes and non-adherence were independently correlated with an increase of HbA1c.45 In the current study, most of the DRP were resolved by the second and third HMR-CP visits as a result of better adherence towards medication and more favourable glycemic control among the patients who received HMR-CP. In other words, significant improvements in patients' adherence and knowledge about diabetes could be the underlying reasons for the improved glycaemic control, as noted in this trial.
It is well acknowledged that diabetic patients require personalised treatment plan in accordance with their daily activities, particularly when lifestyle modifications have failed to control their blood glucose.46 Therefore, home visits under the HMR-CP program can establish a good relationship and trust between the healthcare provider (i.e. community pharmacists) and the diabetic patients. This can further improve the patients' understanding of their medications and subsequently lead to an increased adherence to their medicines. Apart from that, patients will also be comfortable to inform the healthcare provider confidently about their medical problems at home, as compared to the standard healthcare setting in a routine clinic whereby the patients may sometimes feel awkward to share their problems due to the lack of privacy as a result of the busy environment.47
In this RCT, the changes in anthropometric parameters such as weight (p=0.409) and BMI (p=0.575) did not differ significantly between the groups over time. During the HMR-CP visit, the diabetic patients were encouraged to carry out lifestyle modifications such as physical activities to reduce their weight and BMI. However, since most of the patients were more than 60 years old, exercise might be difficult in this age group. This is further strengthened by previous studies that showed a significant negative relationship between age and physical activity level among the Malaysian population.48,49 In another study conducted in Kelantan, the majority of respondents (73.8%) suffered from chronic diseases and also had sedentary lifestyles.50
Furthermore, changes in the utility and MDKT scores pre- and post-intervention were significant between groups (p<0.001). Both utility value and MDKT score increased significantly over time in the HMR-CP group at 6-month follow-up. For the control group, the MDKT score also increased significantly over time. This finding is in line with a previous Malaysian study that investigated the impact of HMR conducted by health clinic pharmacists among diabetes patients.10 Given that the T2DM patients in the HMR-CP group were reported to have better glycaemic control in this trial, this would likely result in an increased QoL. Additionally, a similar observation was noted in a local study that assessed the diabetic patients' QoL following HMR performed by health clinic pharmacists.9
With regard to hospitalisation, diabetic patients are associated with a higher rate of hospitalisation due to the complications of diabetes such as severe dysglycemia.51 However, in this trial, the incidence of hospitalisation was not significantly different between both groups [HMR-CP group, n=3 (3.6%), control group, n=6 (7.2%); p=0.304]. A previous study in Singapore showed that better medication adherence can reduce hospital admission among diabetic patients.52 In another study, elderly patients with a high risk of DRPs showed reduced readmission and emergency visits after receiving HMR from pharmacists.53 However, it is important to note that the incidence of hospitalisation in this study could be under-reported as it was self-reported by the patients.
In the intervention group, HMR-CP significantly increased the patients; PCAR from 0.20 at baseline to 0.51 after 6 months (p<0.001). This was in parallel with a significant reduction in the cost of medication wastage (p = 0.014). The development of HMR-CP has shown an increase in adherence. However, the mean rate of medication adherence in diabetic patients is still far from optimal. At the 6-month of intervention, PCAR is only 51%, and the adherence level of at least 90% was defined as acceptable.54 Pharmacists play an important role in advising patients to reduce medication wastage and to return the excess medication.55 The intervention group was advised by community pharmacists during each home visit to return their remaining medication when collecting their monthly refill at the health clinic. By comparison, the usage of medication by the control group at home could not be monitored. The reduction of medication wastage can help to minimise government expenditure on medication. Even though pharmacists have different ways to eliminate drug waste through the pharmaceutical supply chain, not all are widely implemented.55 The reduction of medication wastage and the subsequent cost saving were in line with a study by Mun (2015) in which medication wastage was reduced by 26.5% after 6 months of HMR.39
Furthermore, greater medication adherence among diabetic patients has been associated with better clinical outcomes as optimum effectiveness of pharmacotherapy can be achieved.56 In this study, the improved adherence rate was in line with other interventional studies (i.e. HMR conducted by health clinic and hospital pharmacists) among diabetic patients.9,10,26,38,39 Similarly, a significant increase in medication adherence was reported among patients who received medication review by CP at the community pharmacies in the UK.57
In terms of medication regime, T2DM patients usually have a complicated regimen.58 Even without the use of insulin, medication compliance can be challenging among them. Of note, the majority of T2DM patients are usually diagnosed with other comorbidities.59 On top of that, uncontrolled blood sugar results in both macro- and micro-vascular complications including blindness, kidney failure, lower limb amputation, stroke, and heart attack.3 Eventually, this translates into an immense impact on the government in terms of finance and human resources in disease treatment and the management of complications.60,61 DRPs are common among patients with a complex medication regimen, especially those with multiple co-morbidities such as dyslipidemia, hypertension, and cardiovascular disease.62 Interventions can be taken in accordance with the identified DRPs (i.e. appropriateness of treatment, adherence toward medications in terms of dosing and timing) in the form of HMR. By implementing HMR, this patient population would greatly benefit from improved health literacy and enhanced medication adherence. Hence, it would result in improved clinical outcomes and the QoL of the patients.
In this RCT, the number of DRP based on the PCNE classification (p<0.001) was significantly reduced across the three time-points. The common DRP reported by patients in the HMR-CP group included suboptimal effects of the drug treatment as a result of non-adherence or taking lower than prescribed dose, inappropriate insulin storage, and inappropriate timing or dosing interval. This is consistent with the previous findings that most diabetic patients experienced problems related to adherence and compliance.63 Some of these problems require attention from the prescriber to modify the dose or type of medication if there are adherence issues or the medication is inappropriate for the patient's condition. By including FMS/MO during HMR sessions, there will be better communication between community pharmacists, FMS/MO, and patients. In short, a collaborative care model that involves healthcare professionals from both public and private settings can further optimise patient care in Malaysia.
In this study, HMR-CP was proven to be the first public-private partnership program in Malaysia that showed a positive impact on certain clinical outcomes. HMR-CP can be the way forward for the MOH, Malaysia in the expansion of the HMR services. Budget allocation should be reserved for the implementation of the HMR service, not unlike other developed countries.43 In Australia, the Diabetes Medication Assistance Services (DMAS) by trained community pharmacists has shown significant health benefits among diabetic patients in terms of improved glycaemic control, reduced risk of non-adherence to medications, and increased knowledge about diabetes self-management.64
There are several limitations to this study. In Malaysia, the prevalence of DM was shown to be the highest among the Indian ethnic group.65 However, there were no Indian participants in this study, likely because Indians account for the lowest percentage of the ethnic population in Kelantan (0.27%).66 Besides, only one health clinic in Kelantan was included in this trial. As such, the findings of this trial may not be generalisable to the entire Malaysian diabetes population. Furthermore, the QoL, knowledge of diabetes, and hospitalisation were self-reported by the patients and thus subjected to bias. In addition, the access to patients' profiles was only limited to the community pharmacists. Therefore, the community pharmacist had to conduct the HMR-CP session with minimal information provided by the researcher. In the future, an integrated online patient database that can be accessed by healthcare professionals including community pharmacists can improve the access to the individual patient's medical needs. There could be social-desirability bias between the control and intervention groups given the different modes of data collection (i.e phone calls versus face-to-face) were used for quality of life and diabetic knowledge. Lastly, future studies can consider more frequent interactions between the community pharmacists and T2DM patients and a shorter follow-up interval rather than every 3 months as proposed in this study.
CONCLUSIONS
Apart from possessing the necessary skills and knowledge, the community pharmacists in Malaysia are also well positioned in the healthcare system to engage further in the provision of care for people with multiple chronic diseases. The 6-month HMR-CP programme showed the feasibility of engaging community pharmacists to provide extended services such as HMR. They can also be incorporated into the collaborative care model that includes FMS/MO in the government sector and community pharmacists in the private sector. Such collaboration will provide a positive impact on the clinical outcomes of diabetic patients. Based on this study, the HMR-CP program significantly improved the T2DM patient's glycaemic control, QoL, medication adherence, and knowledge about diabetes. At the same time, it also reduced the number of DRP and cost of medication wastage. Future studies can explore the economic sustainability of the programme especially with expanded implementation by the MOH.
AUTHOR ROLES (CRediT)
Conceptualization: MRR, CFN, MK.
Data curation: MRR, CFN, MK.
Formal analysis: MRR, CFN, DBW, MK.
Funding acquisition: MRR.
Investigation: MRR, NWH, MM, AR.
Methodology: MRR, CFN, MK.
Project administration: MRR, NWH, MM, AR.
Resources: MRR, CFN, MK.
Supervision: MRR, NWH, MM, AR.
Validation: MRR, CFN, MK.
Writing: original draft: MRR, CFN, DBW, MK.
Writing: review & editing: MRR, CFN, DBW, MK.