Meu SciELO
Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Angiología
versão On-line ISSN 1695-2987versão impressa ISSN 0003-3170
Angiología vol.75 no.5 Madrid Set./Out. 2023 Epub 11-Dez-2023
https://dx.doi.org/10.20960/angiologia.00531
Review
Strategies for the management of steno-occlusive common femoral artery disease. The role of scientific evidence
1Renal Transplantation Unit. Sanatorio de La Trinidad Mitre. Capital Federal, Argentina
2Cardiovascular Surgery and Renal Transplantation Units. Sanatorio de La Trinidad Mitre. Capital Federal, Argentina
Isolated atherosclerotic stenosis or occlusion of the common femoral artery (CFA) is rather uncommon. Traditionally, the standard of care of this entity has been the common femoral artery endarterectomy (CFE).
This manuscript provides a general review of all the current therapeutical strategies (open surgery, endovascular and hybrid approaches) used to treat isolated CFA stenoses or occlusions.
An electronic bibliographic search was performed on the Pubmed and Google databases. Advantages and disadvantages of each revascularization technique were analyzed and the outcomes reported. Common femoral endarterectomy (CFE) showed high technical success and excellent patency rates. However, it was associated with significantly local morbidities, longer lengths of stay and higher medical costs compared with endovascular therapies. Minimally invasive therapeutical approaches showed acceptable outcomes with minor local complications but lower patency rates compared to open surgery.
Further randomized comparative studies are needed to assess the true benefit-risk ratio of traditional CFE vs new minimally invasive therapies for CFA disease.
Keywords: Common femoral artery; Endarterectomy; Endovascular treatment
INTRODUCTION
Although open carotid endarterectomy is considered the standard of care for the management of isolated steno-occlusive lesions of the common femoral artery (CFA), the level of evidence shown in the clinical practice guidelines is weak because of the limited number of randomized controlled trials published. On the other hand, numerous reports suggest that transcatheter endovascular treatments have the potential to replace surgery, especially in certain anatomical situations and lesion characteristics of the CFA (1-4).
The conventional surgical technique has demonstrated low mortality rates and high rates of technical success. One-year primary patency rates between 85 % and 95 % have been reported (5). However, several authors have described a significant incidence rate of local morbidity (infections, hematomas, lymphoceles, etc.), ranging from 8 % to 24 % of the cases, which could potentially change the therapeutic paradigm established for a long time, given the current availability of endovascular and hybrid therapies (6-8).
The objective of this article is to review the current medical literature available on surgical and endovascular therapies, as well as their outcomes in the management of isolated steno-occlusive disease of the CFA. An electronic bibliographic search was conducted in both Spanish and English in PubMed and Google. Keywords used included “common femoral artery,” “femoral endarterectomy,” and “endovascular treatment.” Reviews, case series, and observational, retrospective, and randomized controlled trials published up to 2023 were included.
CURRENT RECOMMENDATIONS OF THE CLINICAL PRACTICE GUIDELINES
Single stenosis or occlusion of the CFA is quite rare. Not many trials on this entity alone are available in the medical literature, and most of them are observational case series, retrospective studies, or single-center registries with low quality cohorts and very limited follow-ups. Similarly, to this date, not too many randomized controlled trials have been published on the short- and long-term results of surgical revascularization compared to endovascular treatment for the management of CFA disease. Therefore, while surgical endarterectomy is considered the standard of care, the level of evidence the guidelines rely on for this recommendation remains weak (level 4; grade C) (5,9,10). The current clinical guidelines on the management of peripheral artery disease (PAD) drafted by the European Society of Cardiology (ESC) do not address the treatment of single CFA lesions exclusively and recommend hybrid approaches combining stenting and endarterectomy or bypass for the initial treatment in cases of associated iliofemoral or femoral disease (superficial femoral/popliteal) (level IIa; grade C) (11). Unlike the European clinical guidelines, the American Heart Association/American College of Cardiology (AHA/ACC) show a preference for the surgical revascularization of lesions involving the CFA, while considering factors associated with technical failure or poor durability after endovascular treatment (12). According to the guidelines recently published by the Canadian Cardiovascular Society on the management of PAD, endovascular therapy is strongly ill-advised for the management of CFA (13).
ANATOMICAL CONSIDERATIONS
The CFA is certainly a challenging vessel to treat. Its anatomical location in a mobile segment, crossing a joint, and close to a bifurcation region is truly challenging. These factors undeniably impact the efficacy and long-term therapeutic outcomes, particularly during transcatheter procedures. Cases of restenosis, stent fracture, or potential loss of vascular access sites for subsequent endovascular procedures have been reported (14-16).
On the other hand, atherosclerotic lesions in these anatomical areas are often extensive, eccentric, and heavily calcified. Additionally, an increased carotid intima-media thickness has been described in these bifurcation areas, especially in smokers, men, and older adults (16,18-20). Considering this anatomical peculiarity, the risk of plaque embolization and the occlusion of efferent vessels, especially the deep femoral artery (DFA), can happen (16). The importance of keeping the DFA patency should never be underestimated, especially regarding the management of critical limb ischemia. Former studies have shown that effective DFA perfusion is essential to preserve the knee joint function and achieve a high degree of subsequent functional rehabilitation (21). In these cases, percutaneous revascularization should be performed with utmost care to preserve the DFA patency and, in open cases, avoid its occlusion.
Isolated deep femoral endarterectomy has also proven to be an effective and durable therapeutic revascularization strategy, especially in high-risk patients with previous vascular reconstructions and extensive popliteal and superficial disease. Significant clinical improvement has been achieved in many cases, leading to successful salvage of the diseased lower limb (21-25).
CURRENT TREATMENT OPTIONS. RESULTS
Femoral endarterectomy
Conventional surgery has been the standard of care for steno-occlusive disease of the CFA for over 50 years. Because this artery is easily accessible to perform surgical procedures, most surgeons decide to treat the disease with endarterectomy, with or without patching. It is considered a technically straightforward and short-duration procedure (9,26).
Undoubtedly, this surgical approach is quite effective to alleviate symptoms in patients with moderate and severe claudication (Rutherford 2 and 3), rest pain or ischemic ulceration (Rutherford 4, 5, and 6), with high rates of limb survival and long-term patency (27-33). According to the medical literature available, 1- and 5-year primary patency rates range from 82 % to 93 % and 74 % to 94 %, respectively, with acceptable long-term durability (9,29,30). The reasons for such excellent outcomes are, probably, larger vessel diameters, short lengths, and high-velocity flows (29).
However, mortality and morbidity are not insignificant and should be taken into consideration. Although the occurrence of cardiovascular, renal, or pulmonary complications is relatively low, these events are followed by postoperative mortality rates of 0 % to 4 % (29,30,34-37). Kechagias et al. and Nguyen et al. described perioperative mortality rates of 2 % and 3.4 % (even up to 15 %), respectively, for postoperative morbidity and mortality combined (26,33).
The presence of comorbidities in these patients, such as overweight, female sex, diabetes mellitus, dialysis, and chronic steroid use, has been identified as a positive predictor of such postoperative morbidity (26).
The most common postoperative complications seen are those associated with both the approach and the wound (superficial or deep infections, dehiscences, hematomas, seromas, pseudoaneurysms, hemorrhages, thrombosis, etc.), with rates reported in 8 % to 24 % of the cases (26,33,36,37). A retrospective study including 111 CFA endarterectomies (n = 90 patients) reported nearly a third of local surgical site complications (17.1 %, infections; 9 %, hematomas; and 5.4 %, seromas). A different series published by Cordon et al. described a 22 % rate of local morbidity (36-38).
The most relevant risk factors associated with the presence of such local complications seem to be a previous inguinal approach and the use of wound drainage (37).
Vein patch has been considered the material of choice for many years thanks to its infection-resistant properties. However, no significant association between the type of patch used (synthetic, bovine, or venous) and the occurrence of surgical site infections has ever been demonstrated (37,39-42).
On the other hand, the current medical literature available suggests that most of these postoperative complications occur after hospital discharge. In a large cohort study (n = 1843 CFA endarterectomies), an 8.4 % rate of local complications was reported, 86 % of which occurred after hospital admission (26). Similarly, Derksen et al. reported a 14 % rate of local morbidity, with 95 % of the cases occurring within the first 30 days after surgery (37).
In some cases, surgical re-exploration may be indicated in the presence of some local complications. The main reasons given for such an indication are bleeding, thrombosis, and pseudoaneurysm, with rates between 5 % and 6.5 % (9,26,33,37).
All these adverse circumstances result in long lengths of stay and significant financial burdens that impact not only the health care systems (hospital visits, additional antibiotic usage, home health care services, hospital readmissions, and additional procedures) but also patients themselves (longer downtimes, higher transportation and drug costs) (43-46).
Endovascular therapy
Over the past few years, and following the ongoing advancements made in the technological field, significant shifts toward the paradigm of endovascular therapy have been observed. Because of the minimally invasive nature of these new procedures, the use of endovascular therapies for the management of the CFA has been associated with shorter lengths of stay, fast recoveries, lower morbidity/mortality rates (5.5 % and 3.1 %, on average, respectively), and technical success rates of nearly 93.3 %. A 2-year cumulative primary patency rate of nearly 80 % has been reported (1,14,15,47).
Different techniques have been proposed and results have been published, ranging from plain old balloon angioplasty to atherectomy. However, significant aspects should be analyzed here (48-53).
The main concern on the use endovascular procedures is the patency rate and need for mid-term reinterventions. Studies demonstrate that the rate of success varies considerably based on the specific anatomical location and intrinsic characteristics of the lesion (that can be categorized as simple when the CFA bifurcation is not compromised (type 1 and 2 lesions), or complex if such bifurcation is compromised, or associated with other anatomical lesions (type 3 and 4 lesions) (4,16,17) (Fig. 1).

Source: Azéma L, Davaine JM, Guyomarch B, et al. Reparación endovascular de la arteria femoral común y lesiones arteriales concomitantes. Eur J Vasc Endovasc Surg 2011;41(6):787-93. DOI: 10.1016/j.ejvs.2011.02.025
Figure 1. Classification of occlusive disease of the common femoral artery. CFA lesions can be categorized as simple if the CFA bifurcation is not compromised (A: type I lesion; B: type II lesion), or complex if the CFA bifurcation is compromised or associated with other anatomical lesions (C: type III lesion; D: type IV lesion).
An extensive review of the current medical literature available on lesions treated only with angioplasty reveals 1-year primary patency rates from 59 % to 88 %, and 2-, 4-, and 5-year patency rates of 83 %, 66 %, and 60 %, respectively (8,54-57). These suboptimal outcomes could be associated with the eccentric and calcified nature of the lesion, which in some cases requires higher balloon pressures and shear forces, with the corresponding risk of intimal tear and media layer dissection (8,58). Stenting has been used in the context of these suboptimal technical results or as a bailout procedure after failed angioplasties. On the other hand, the patency of the combined approach (angioplasty/stent) has shown better results compared with angioplasty alone (4,59-65). Paris et al. reported on 26 cases of CFA lesions treated with stent-assisted angioplasty, demonstrating 1-year primary patency rates of nearly 93 %. Other studies have reported 2- and 5-year patency rates of 92.5 % and 72.5 %, respectively (60).
However, caution is advised when using stents at CFA level. Cases of restenosis, fractures, DFA involvement, or limitation of future vascular accesses could be associated with this technique.
The overall incidence of restenosis after the endovascular management of the CFA could be explained by the calcified nature of CFA lesions that, in the mid-term, may be a beneficial factor to induce a less severe inflammatory response to endovascular aggression (15,66).
Several authors suggest that procedures involving bifurcations (type 3 and 4 lesions) are associated with the highest rate of restenosis (4,16). Boufi et al. described a rate of restenosis rate of nearly 22 % in complex lesion groups (66). Similarly, Azema et al. described 1-year rates of restenosis of nearly 20 % (16). Another case series including 33 limbs surgically treated to treat atherosclerotic disease at CFA level showed 1- and 2-year rates of restenosis of 13 % and 17 %, respectively (15).
Conversely, the risk of stent fracture appears to be higher in type 1 lesions located just above the inguinal segment (16,59,60). A case series of 53 CFA procedures (n = 48 patients) found a total of 4 stent fractures (7.5 %) at the 2-year follow-up. Azema et al. reported only 1 case of stent fracture in their study (n = 36 patients; n = 43 stent deployments) at the 22-month follow-up, while Stricker et al. reported that the only stent fracture found in their study (n = 27 patients; n = 33 stent deployments/PTAs) occurred in a patient with a balloon-expandable stent graft at a median follow-up of 23 months (15,16). Based on the current medical literature available, it has been suggested that the use of short, self-expanding nitinol stents is less prone to fracture due to their greater radial strength and crush resistance (67-69). The VMI-CFA (ClinicalTrials.gov / ID: NCT02804113), a prospective, multicenter trial that assessed the effectiveness of the self-expanding Supera nitinol peripheral stent (Abbott Vascular, Santa Clara, CA, United States) to treat CFA stenosis or occlusion showed acceptable results in terms of patency, lesion revascularization, and significant clinical benefits at the 24-month follow-up. This study suggests the safety and feasibility profiles of these types of stents (70).
The risk of compromising the DFA flow would be higher in type 2 lesions and, especially, type 3 lesions at the bifurcation area. In fact, some authors recommend catheterizing the DFA first while keeping a guidewire in the artery throughout the entire procedure (17).
Another aspect that should be taken into consideration is the potential risk to the vascular access regarding future surgeries and stenting. However, there have been no related cases to this date. It just seems to be a theoretical concern. Given the limited literature available and lack of substantial data, further studies would be required to obtain well-supported evidence (8,15,16,59).
Undoubtedly, the strategy used in the endovascular reconstruction of CFA bifurcations and, especially, in the preservation of the DFA patency is challenging. The application of the “1-stent technique” has shown good results. DFA involvement has been reported in only 9 % of the cases (71-73). The use of the “2-stent technique” as an alternative approach has also shown promising results (73,74). However, these findings are based on a small number of patients and follow-ups. More trials are needed before drawing reliable and definitive conclusions.
Other ways of treating CFA lesions are currently being studied. Atherectomy (AT) devices, combined with percutaneous transluminal angioplasty (PTA) or drug-coated balloons, could be a promising option to treat more complex lesions (77,78). Although several reports have demonstrated the role played by AT to treat femoropopliteal disease, very few studies have analyzed the use of this technique to treat CFA disease alone. Dattilo et al. reported 1-year primary patency rates of 88 % and 2- and 3-year rates of 83 % (63).
Another study that compared AT/PTA with PTA alone reported 20-month primary patency rates of 86 % and 70.7 %, respectively (55). Better results were seen with the combined use of AT and drug-coated balloons, with 1-year primary patency rates of nearly 90 % (76). The safety of this combined technique would be associated with an improved lumen size and effective reduction of eccentric lesions, avoiding stretch-induced injuries and arterial wall dissections before using the PTA or the drug-coated balloon, which would also enhance drug uptake. However, the risk of distal embolization remains a significant drawback associated with AT, which is why the use of embolic protection devices is advised (77). Further studies are needed to define safety, efficacy, and short- and long-term outcomes.
RANDOMIZED CONTROLLED TRIALS
To date, there aren't many randomized controlled trials (RCTs) comparing open surgical approaches to minimally invasive ones to treat atherosclerotic disease of the CFA without an associated anatomical lesion. Study protocols are shown in table I (78-82).
Table I. Randomized controlled trials. Study protocols.
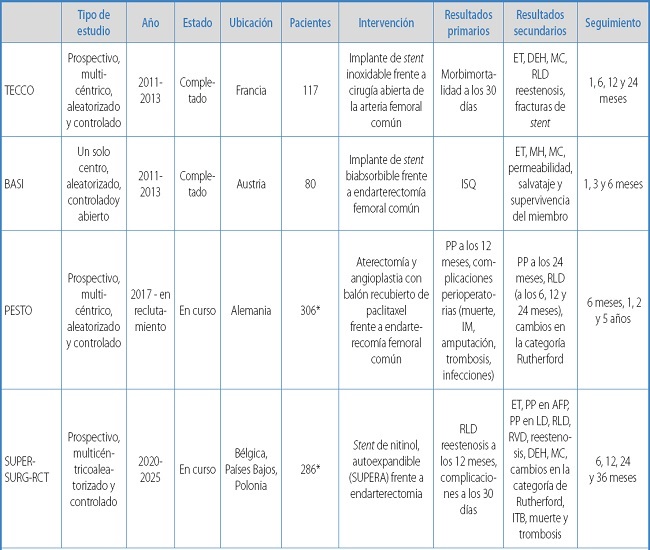
*Estimated;CI: clinical improvement; DFA: deep femoral artery; HI: hemodynamic improvement; LOS: length of stay; PP: primary patency; SSI: surgical site infection; TLR: target lesion revascularization; TS: technical success; TVR: target vessel revascularization.
Gouëffic et al.'s TECCO trial compared the outcomes of open surgery with those of endovascular treatment with self-expanding stents to treat atherosclerotic lesions to the CFA. The authors saw that the perioperative morbidity/mortality rate and mean length of stay after stenting were significantly lower compared to surgery. However, there were no differences regarding survival, lesion revascularization, limb revascularization, and primary patency at 2 years. Certainly, they support a significant benefit based on morbidity and mortality outcomes (79).
Linni et al.'s BASI trial compared the effectiveness of bioresorbable stent implantation to common femoral endarterectomy, reporting a higher number of surgical site infections with conventional surgical treatment compared to endovascular treatment. Lower patency rates were reported with the endovascular treatment and significantly different rates of failure after endovascular treatment. Finally, they concluded that the use of stents does not seem to be a feasible option for CFA occlusion and is very limited in cases of CFA stenosis (80). The results of 2 upcoming RCTs will shed more light on the application of these new therapies compared to classic femoral endarterectomy.
The PESTO is a prospective multicenter clinical trial to assess the effectiveness of a combined approach of atherectomy and paclitaxel-coated balloon angioplasty to treat CFA disease. This study will enroll nearly 306 participants, who will be randomized and followed for a total of 24 months (81).
The ongoing SUPERSURG - RCT is another multicenter controlled clinical trial that will assess the safety and efficacy profile of endovascular treatment with the Supera stent (Abbott Vascular, Santa Clara, CA, United States) compared to surgical endarterectomy in patients with stenotic or restenotic CFA lesions. A total of 286 patients will be enrolled and randomized on a 1:1 ratio with 1-, 6-, 12-, 24-, and 36-month follow-ups (82).
HYBRID THERAPY
The combined use of open and endovascular techniques in a single surgical procedure (hybrid procedures) has gained significance over the past few years. In these cases, the CFA plays a key role. In the presence of stenotic or occlusive lesions of the CFA associated with concomitant and significant afferent or efferent disease, the hybrid approach provides not only the proven durability of femoral endarterectomy but also excellent surgical access for endovascular treatment, allowing us to treat extensive lesions, especially in high-risk patients (83-87). On the other hand, therapeutic delay time in cases of complete revascularization due to critical limb ischemia would reduce significantly. The technical failure of the hybrid procedure would not negatively impact the need for future additional revascularization (85).
Similarly, simultaneous hybrid procedures have yielded significant benefits associated with fewer major local complications, lower costs, and shorter lengths of stays compared with traditional 2-stage surgeries (86,87).
A significant number of series published suggest the safety and efficacy profile of these procedures (72,74,84). Mid- and long-term results seem encouraging. Primary (PP) and secondary patency (SP) rates over 85 % and 95 % at 2 and 5 years, respectively, have been reported, along with acceptable technical success and low morbidity/mortality rates (85,90). However, most of these studies include limited, heterogeneous patient cohorts with a wide variety of open and endovascular procedures, which added to the different reporting standards and follow-up, complicate making comparisons and drawing definitive conclusions. Many authors have reported patency rates separately for different reconstruction sites and suggest lower patency rates at the endovascular segment (72,74,84). Very few authors have considered the two patency rates combined, which would be more useful to assess outcomes (81-85,87).
A recent prospective randomized trial conducted by Starodubtsevis et al. compared the short- and mid-term safety and efficacy profile of hybrid repair (iliac stenting + CFE and patch angioplasty) and open reconstruction (aortofemoral bypass + CFE) in patients with coexisting occlusive disease of the iliac and common femoral arteries (CFA). The PP and SP rates reported were 93 % and 91 %, which is similar to the 99 % and 98 % rates reported at 12 and 36 months, respectively. Significantly shorter lengths of stay and less perioperative morbidity were seen with the hybrid procedure.
CONCLUSIONS
The isolated disease of the CFA is extremely rare, and its treatment remains a topic of discussion. This review suggests that scientific evidence-based decision-making is still limited. The medical literature available mainly includes comparative and retrospective descriptive trials, case series, cohort studies, and systematic reviews. However, few RCTs have been published to date, and more are expected. While open femoral endarterectomy is known to have high technical success and excellent short- and long-term patency rates, it is associated with significant local morbidity. Endovascular therapeutic strategies have been proposed with acceptable results. However, longer-term research is still needed to better define durability and address concerns associated with the type of stent used, the risk of technical failure, stent fracture, or loss of future vascular access. To guarantee that guideline recommendations remain updated, new data should be evaluated to identify potential qualitative characteristics of CFA lesions and determine the best candidate for each strategy. While we await the results of more studies and RCTs, decision-making should be based on clinical and anatomical characteristics, patient preferences, and associated comorbidities.
Nigro B, Ferrari-Ayarragaray JE. Strategies for the management of steno-occlusive common femoral artery disease. The role of scientifi c evidence. Angiología 2023;75(5):298-308
REFERENCES
1. Bonvini RF, Rastan A, Sixt S, et al. Endovascular treatment of common femoral artery disease: medium-term outcomes of 360 consecutive procedures. J Am Coll Cardiol 2011;58:792-8. DOI: 10.1016/j.jacc.2011.01.070 [ Links ]
2. Bonvini RF, Rastan A, Sixt S, et al. Angioplasty and provisional stent treatment of common femoral artery lesions. J Vasc Interv Radiol 2013;24:175-83. DOI: 10.1016/j.jvir.2012.10.020 [ Links ]
3. Stricker H, Jacomella V. Stent-assisted angioplasty at the level of the common femoral artery bifurcation: midterm outcomes. J Endovasc Ther 2004;11:281-6. [ Links ]
4. Nasr B, Kaladji A, Vent PA, et al. Long-Term Outcomes of Common Femoral Artery Stenting. Ann Vasc Surg 2017;40:10-8. DOI: 10.1016/j.avsg.2016.07.088 [ Links ]
5. Ballotta E, Gruppo M, Mazzalai F, et al. Common femoral artery endarterectomy for occlusive disease: an 8-year single-center prospective study. Surgery 2010;147(2):268-74. DOI: 10.1016/j.surg.2009.08.004 [ Links ]
6. Kim JH, So BJ, Byun SJ, et al. The Advantage of Common Femoral Endarterectomy Alone or Combined with Endovascular Treatment. Vasc Specialist Int 2018;34(3):65-71. DOI: 10.5758/vsi.2018.34.3.65 [ Links ]
7. Pecoraro F, Pakeliani D, Bruno S, et al. Simultaneous Hybrid Treatment of Multilevel Peripheral Arterial Disease in Patients with Chronic Limb-Threatening Ischemia. J Clin Med 2021;10(13):2865. DOI: 10.3390/jcm10132865 [ Links ]
8. Wong G, Lahsaei S, Aoun J, et al. Management of common femoral artery occlusive disease: A review of endovascular treatment strategies and outcomes. Catheter Cardiovasc Interv 2019;93(3):514-21. DOI: 10.1002/ccd.27983 [ Links ]
9. Kang JL, Patel VI, Conrad MF, et al. Common femoral artery occlusive disease: contemporary results following surgical endarterectomy. J Vasc Surg 2008;48(4):872-7. DOI: 10.1016/j.jvs.2008.05.025 [ Links ]
10. Setacci C, de Donato G, Teraa M, et al. Chapter IV: Treatment of critical limb ischaemia. Eur J Vasc Endovasc Surg 2011;42(Suppl.2):S43-59. DOI: 10.1016/S1078-5884(11)60014-2 [ Links ]
11. Aboyans V, Ricco JB, Bartelink MEL, et al. ESC Scientific Document Group. 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS): Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteriesEndorsed by: the European Stroke Organization (ESO)The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur Heart J 2018;39(9):763-816. DOI: 10.1093/eurheartj/ehx095 [ Links ]
12. Kithcart AP, Beckman JA. ACC/AHA versus ESC Guidelines for Diagnosis and Management of Peripheral Artery Disease: JACC Guideline Comparison. J Am Coll Cardiol 2018;72(22):2789-801. DOI: 10.1016/j.jacc.2018.09.041 [ Links ]
13. Primary Panel;Abramson BL, Al-Omran M, Anand SS, et al. Canadian Cardiovascular Society 2022 Guidelines for Peripheral Arterial Disease. Can J Cardiol 2022;38(5):560-87. DOI: 10.1016/j.cjca.2022.02.029 [ Links ]
14. Boufi M, Ejargue M, Gaye M, et al. Systematic review and meta-analysis of endovascular versus open repair for common femoral artery atherosclerosis treatment. J Vasc Surg 2021;73(4):1445-55. DOI: 10.1016/j.jvs.2020.10.026 [ Links ]
15. Stricker H, Jacomella V. Stent-assisted angioplasty at the level of the common femoral artery bifurcation: midterm outcomes. J Endovasc Ther 2004;11:281-6. [ Links ]
16. Azéma L, Davaine JM, Guyomarch B, et al. Endovascular repair of common femoral artery and concomitant arterial lesions. Eur J Vasc Endovasc Surg 2011;41(6):787-93. DOI: 10.1016/j.ejvs.2011.02.025 [ Links ]
17. López JF, Magne JL, Champetier J. The femoral artery and flexion of the hip joint. Surg Radiol Anat 1989;11(4):275e81. [ Links ]
18. Lim CS, Gohel MS, Shepherd AC, et al. Iliac artery compression in cyclists: mechanisms, diagnosis and treatment. Eur J Vasc Endovasc Surg 2009;38(2):180-6. DOI: 10.1016/j.ejvs.2009.03.024 [ Links ]
19. Gogineni A, Ravigururajan TS. Flow Dynamics and Wall Shear Stresses in a Bifurcated Femoral Artery. J Biomed Eng Med Devic 2017;2(3). [ Links ]
20. Kornet L, Lambregts J, Hoeks AP, et al. Differences in near-wall shear rate in the carotid artery within subjects are associated with different intima-media thicknesses. Arterioscler Thromb Vasc Biol 1998;18(12):1877-84. DOI: 10.1161/01.atv.18.12.1877 [ Links ]
21. Boren CH, Towne JB, Bernhard VM, et al. Profundapopliteal collateral index. A guide to successful profundaplasty. Arch Surg 1980;115(11):1366-72. DOI: 10.1001/archsurg.1980.01380110098015 [ Links ]
22. Rollins DL, Towne JB, Bernhard VM, et al. Isolated profundaplasty for limb salvage. J Vasc Surg 1985;2(4):585-90. DOI: 10.1067/mva.1985.avs0020585 [ Links ]
23. Morris GC Jr, Edwards E, Cooley DA, et al. Surgical importance of profunda femoris artery. Analysis of 102 cases with combined aortoiliac and femoropopliteal occlusive disease treated by revascularization of deep femoral artery. Arch Surg 1961;82:32-7. DOI: 10.1001/archsurg.1961.01300070036005 [ Links ]
24. Mills JL, Taylor SM, Fujitani RM. The role of the deep femoral artery as an inflow site for infrainguinal revascularization. J Vasc Surg 1993;18(3):416-23. DOI: 10.1067/mva.1993.48445 [ Links ]
25. Goldstone J, Malone JM, Moore WS. Importance of the profunda femoris artery in primary and secondary arterial operations for lower extremity ischemia. Am J Surg 1978;136(2):215-20. DOI: 10.1016/0002-9610(78)90231-3 [ Links ]
26. Nguyen BN, Amdur RL, Abugideiri M, et al. Postoperative complications after common femoral endarterectomy. J Vasc Surg 2015;61(6):1489-94.e1. DOI: 10.1016/j.jvs.2015.01.024 [ Links ]
27. McGovern PJ Jr, Stark KR, Kaufman JL, et al. Management of common femoral artery occlusion: a report of ten cases. J Cardiovasc Surg 1987;28:38-41. [ Links ]
28. Archie JP Jr, Feldtman RW. Common femoral artery atherosclerotic occlusion. Difficult to diagnose but easy to treat. Am Surg 1982;48:339-40. [ Links ]
29. Mukherjee D, Inahara T. Endarterectomy as the procedural of choice for atherosclerotic occlusive lesions of the common femoral artery. Am J Surg 1989;157:498-500. [ Links ]
30. Springhorn ME, Kinney M, Littooy FN, et al. Inflow atherosclerotic disease localized to the common femoral artery: treatment and outcome. Ann Vasc Surg 1991;5:234-40. [ Links ]
31. Nelson PR, Powell RJ, Schermerhorn ML, et al. Early results of external iliac artery stenting combined with common femoral artery endarterectomy. J Vasc Surg 2002;35:1107-13. [ Links ]
32. Savolainen H, Hanse A, Diehm N, et al. Small is beautiful: why profundaplasty should not be forgotten. World J Surg 2007;31:2058-61. [ Links ]
33. Kechagias A, Ylonen K. Long-term outcome after isolated endarterectomy of the femoral bifurcation. World J Surg 2008;32:51-4. [ Links ]
34. Van Himbeeck FJ, van Knippenberg LA, Niessen MC, van Griethuysen AJ. Wound infection after arterial surgical procedures. Eur J Vasc Surg 1992;6:494-8. [ Links ]
35. Stewart AH, Eyers PS, Earnshaw JJ. Prevention of infection in peripheral arterial reconstruction: a systematic review and meta-analysis. J Vasc Surg 2007;46:148-55. [ Links ]
36. Cordon A, Aillet S, Jarno P, et al. Endarterectomy of the femoral tripod: long-term results and analysis of failure factors. Ann Chir 2001;126:777-82. [ Links ]
37. Derksen WJM, Verhoeven BAN, van de Mortal RHW, et al. Risk factors for surgical-site infection following common femoral artery endarterectomy. Vasc Endovasc Surg 2009;43:69-75. [ Links ]
38. Ballotta E, Gruppo M, Mazzalai F, et al. Common femoral artery endarterectomy for occlusive disease: an 8-year single-center prospective study. Surgery 2010;147(2):268-74. DOI: 10.1016/j.surg.2009.08.004 [ Links ]
39. Edwards WH Jr, Martin RS III, Jenkins JM, et al. Primary graft infections. J Vasc Surg. 1987;6:235-9. [ Links ]
40. Szilagyi DE, Smith RF, Elliott JP, et al. Infection in arterial reconstruction with synthetic grafts. Ann Surg 1972;176:321-3. [ Links ]
41. Van Himbeeck FJ, van Knippenberg LA, Niessen MC, et al. Wound infection after arterial surgical procedures. Eur J Vasc Surg 1992;6:494-8. [ Links ]
42. Stewart AH, Eyers PS, Earnshaw JJ. Prevention of infection in peripheral arterial reconstruction: a systematic review and meta-analysis. J Vasc Surg 2007;46:148-55. [ Links ]
43. Boltz MM, Hollenbeak CS, Julian KG, et al. Hospital costs associated with surgical site infections in general and vascular surgery patients. Surgery 2011;150(5):934-42. DOI: 10.1016/j.surg.2011.04.006 [ Links ]
44. Dimick JB, Chen SL, Taheri PA, et al. Hospital costs associated with surgical complications: a report from the private-sector National Surgical Quality Improvement Program. J Am Coll Surg 2004;199:531-7. [ Links ]
45. Khan NA, Quan H, Bugar JM, et al. Association of postoperative complications with hospital costs and length of stay in a tertiary care center. J Gen Intern Med 2006;21:177-80. [ Links ]
46. Davenport DL, Henderson WG, Khuri SF, et al. Preoperative risk factors and surgical complexity are more predictive of costs than postoperative complications: a case study using the National Surgical Quality Improvement Program (NSQIP) database. Ann Surg 2005;242:463-8;discussion 468-71. [ Links ]
47. Soga Y, Tomoi Y, Sato K, et al. Clinical outcome after endovascular treatment for isolated common femoral and popliteal artery disease. Cardiovasc. Interv Ther 2013;28:250-7. [ Links ]
48. Wong G, Lahsaei S, Aoun J, et al. Management of common femoral artery occlusive disease: A review of endovascular treatment strategies and outcomes. Catheter Cardiovasc Interv 2019;93(3):514-21. DOI: 10.1002/ccd.27983 [ Links ]
49. Imran HM, Hyder ON, Soukas PA. Efficacy and safety of adjunctive drug-coated balloon therapy in endovascular treatment of common femoral artery disease. Cardiovasc Revasc Med 2019;20:210-4. [ Links ]
50. Baumann F, Ruch M, Willenberg T, et al. Endovascular treatment of common femoral artery obstructions. J Vasc Surg 2011;53:1000-6. [ Links ]
51. Siracuse JJ, Van Orden K, Kalish JA, et al. Endovascular treatment of the common femoral artery in the Vascular Quality Initiative. J Vasc Surg 2017;65:1039-46. [ Links ]
52. Morris-Stiff G, Moawad M, Appleton N, et al. Long-term clinical outcome following lower limb arterial angioplasty. Ann R Coll Surg Engl 2011;93:250-4. [ Links ]
53. Kuo TT, Chen PL, Huang CY, et al. Outcome of drug-eluting balloon angioplasty versus endarterectomy in common femoral artery occlusive disease. J Vasc Surg 2019;69:141-7. [ Links ]
54. Dormandy JA, Rutherford RB. Management of peripheral arterial disease (PAD). TASC working group. Trans Atlantic inter society consensus (TASC). J Vasc Surg 2000;31:S1-S296. [ Links ]
55. Mehta M, Zhou Y, Paty PS, et al. Percutaneous common femoral artery interventions using angioplasty, atherectomy, and stenting. J Vasc Surg 2016;64(2):369-79. DOI: 10.1016/j.jvs.2016.03.418 [ Links ]
56. Katzen BT, Chang J, Knox WG. Percutaneous transluminal angioplasty with the Grüntzig balloon catheter: A review of 70 cases. Arch Surg 1979;114:1389-99. [ Links ]
57. Johnston KW, Rae M, Hogg-Johnston SA, et al. 5-year results of a prospective study of percutaneous transluminal angioplasty. Ann Surg 1987;206(4):403-13. DOI: 10.1097/00000658-198710000-00002 [ Links ]
58. Fitzgerald PJ, Ports TA, Yock PG. Contribution of localized calcium deposits to dissection after angioplasty. An observational study using intravascular ultrasound. Circulation 1992;86:64-70. [ Links ]
59. De Blic R, Deux JF, Kobeiter H, et al. Initial experience with percutaneous angioplasty of the common femoral artery in de novo stenotic lesions. Ann Vasc Surg 2015;29:1493-500. [ Links ]
60. Paris CL, White CJ, Collins TJ, et al. Catheter-based therapy of common femoral artery atherosclerotic disease. Vasc Med 2011;16:109-12. [ Links ]
61. Baerlocher MO, Kennedy SA, Rajebi MR, et al. Meta-analysis of drug eluting balloon angioplasty and drug-eluting stent placement for infrainguinal peripheral arterial disease. J Vasc Interv Radiol 2015;26:459-73. [ Links ]
62. Thiney PO, Millon A, Boudjelit T, et al. Angioplasty of the common femoral artery and its bifurcation. Ann Vasc Surg 2015;29:960-7. [ Links ]
63. Dattilo PB, Tsai TT, Kevin Rogers R, et al. Acute and mediumterm outcomes of endovascular therapy of obstructive disease of diverse etiology of the common femoral artery. Catheter Cardiovasc Interv 2013;81:1013-22. [ Links ]
64. Baumann F, Ruch M, Willenberg T, et al. Endovascular treatment of common femoral artery obstructions. J Vasc Surg 2011;53(4):1000-6. DOI: 10.1016/j.jvs.2010.10.076 [ Links ]
65. Laird JR. Endovascular treatment of common femoral artery disease: Viable alternative to surgery or just another short-term fix. J Am Coll Cardiol 2011;58:799-800. [ Links ]
66. Boufi M, Ejargue M, Gaye M, et al. Systematic review and meta-analysis of endovascular versus open repair for common femoral artery atherosclerosis treatment. J Vasc Surg 2021;73(4):1445-55. DOI: 10.1016/j.jvs.2020.10.026 [ Links ]
67. Linni K, Ugurluoglu A, Hitzl W, et al. Bioabsorbable Stent Implantation vs. Common Femoral Artery Endarterectomy: Early Results of a Randomized Trial. J Endovasc Ther 2014;21:493-502. [ Links ]
68. Calligaro K, Balraj P, Moudgill N, et al. Results of polytetrafluoroethylene-covered nitinol stents crossing the inguinal ligament. J Vasc Surg 2013;57(2):421-6. [ Links ]
69. Neglén P, Tackett TP Jr, Raju S. Venous stenting across the inguinal ligament. J Vasc Surg 2008;48:1255-61. [ Links ]
70. Deloose K, et al. A Study Evaluating the Endovascular Treatment of Subjects With Stenotic or Restenotic Lesions of the Common Femoral Artery With the Supera Vascular Mimetic Implant Compared to Surgical Common Femoral Artery Endarterectomy (SUPERSURG-RCT). 2023. ClinicalTrials.gov [ Links ]
71. Yamawaki M, Hirano JK, Nakano M, et al. Deployment of Self-Expandable Stents for Complex Proximal Superficial Femoral Artery Lesions Involving the Femoral Bifurcation With or without Jailed Deep Femoral Artery. Catheterization and Cardiovascular Interventions 2013;81:1031-41. [ Links ]
72. Hong SJ, Ko YG, Suh Y, et al. Outcomes of stents covering the deep femoral artery origin. EuroIntervention 2014;10:632-9. [ Links ]
73. Mathisen SR, Akesson M, Lindh M, et al. Kissing stents in the common femoral artery bifurcation for critical limb ischemia: technical description and report of three cases. Vascular 2007;15(4):211-4. DOI: 10.2310/6670.2007.00037 [ Links ]
74. Thiney PO, Millon A, Boudjelit T, et al. Angioplasty of the common femoral artery and its bifurcation. Ann Vasc Surg 2015;29(5):960-7. DOI: 10.1016/j.avsg.2015.02.001 [ Links ]
75. Stavroulakis K, Schwindt A, Torsello G, et al. Directional atherectomy with antirestenotic therapy vs drug-coated balloon angioplasty alone for common femoral artery atherosclerotic disease. J Endovasc Ther 2017;25:92-9. [ Links ]
76. Cioppa A, Stabile E, Salemme L, et al. Combined use of directional atherectomy and drug-coated balloon for the endovascular treatment of common femoral artery disease: immediate and one-year outcomes. EuroIntervention 2017;12:1789e94. [ Links ]
77. Zeller T, Langhoff R, Rocha-Singh KJ, et al. Directional atherectomy followed by a Paclitaxel-coated balloon to Inhibit restenosis and Maintain vessel patency: Twelve-month results of the DEFINITIVE AR study. Circ Cardiovasc Interv 2017;10. [ Links ]
78. Starodubtsev V, Mitrofanov V, Ignatenko P, et al. Editor's Choice - Hybrid vs. Open Surgical Reconstruction for Iliofemoral Occlusive Disease: A Prospective Randomised Trial. Eur J Vasc Endovasc Surg. 2022 Apr;63(4):557-565. DOI: 10.1016/j.ejvs.2022.02.002 [ Links ]
79. Gouëffic Y, Della Schiava N, Thaveau F, et al. Stenting or Surgery for De Novo Common Femoral Artery Stenosis. JACC Cardiovasc Interv 2017;10(13):1344-54. DOI: 10.1016/j.jcin.2017.03.046 [ Links ]
80. Linni K, Ugurluoglu A, Hitzl W, et al. Bioabsorbable stent implantation vs. common femoral artery Endarterectomy: Early Results of a randomized trial. J Endovasc Ther 2014;21(4):493-502. DOI: 10.1583/14-4699R.1 [ Links ]
81. Rastan A, Zeller T, et al Percutaneous Intervention Versus Surgery in the Treatment of Common Femoral Artery Lesions (PESTO-AFC). ClinicalTrials.gov. [ Links ]
82. Deloose K, et al. A Study Evaluating the Endovascular Treatment of Subjects With Stenotic or Restenotic Lesions of the Common Femoral Artery With the Supera Vascular Mimetic Implant Compared to Surgical Common Femoral Artery Endarterectomy (SUPERSURG-RCT). 2023. ClinicalTrials.gov [ Links ]
83. Guo J, Guo L, Tong Z, et al. Directional Atherectomy Is Associated with Better Long-Term Efficiency Compared with Angioplasty for Common Femoral Artery Occlusive Disease in Rutherford 2-4 Patients. Ann Vasc Surg 2018;51:65-71. DOI: 10.1016/j.avsg.2017.12.004 [ Links ]
84. Dosluoglu HH, Lall P, Cherr GS, et al. Role of simple and complex hybrid revascularization procedures for symptomatic lower extremity occlusive disease. J Vasc Surg 2010;51(6):1425-35.e1. DOI: 10.1016/j.jvs.2010.01.092 [ Links ]
85. Nelson PR, Powell RJ, Schermerhorn ML, et al. Early results of external iliac artery stenting combined with common femoral artery endarterectomy. J Vasc Surg. 2002;35(6):1107-13. DOI: 10.1067/mva.2002.124374 [ Links ]
86. Ebaugh JL, Gagnon D, Owens CD, et al. Comparison of costs of staged versus simultaneous lower extremity arterial hybrid procedures. Am J Surg 2008;196(5):634-40. DOI: 10.1016/j.amjsurg.2008.08.003 [ Links ]
87. Antoniou GA, Sfyroeras GS, Karathanos C, et al. Hybrid endovascular and open treatment of severe multilevel lower extremity arterial disease. Eur J Vasc Endovasc Surg 2009;38(5):616-22. DOI: 10.1016/j.ejvs.2009.06.016 [ Links ]
88. Matsagkas M, Kouvelos G, Arnaoutoglou E, et al. Hybrid procedures for patients with critical limb ischemia and severe common femoral artery atherosclerosis. Ann Vasc Surg 2011;25(8):1063-9. DOI: 10.1016/j.avsg.2011.07.010 [ Links ]
89. Balaz P, Rokosny S, Wohlfahrt P, et al. Early and late outcomes of hybrid endovascular and open repair procedures in patients with peripheral arterial disease. Vasa 2013;42(4):292-300. DOI: 10.1024/0301-1526/a000290 [ Links ]
90. Kashyap VS, Pavkov ML, Bena JF, et al. The management of severe aortoiliac occlusive disease: endovascular therapy rivals open reconstruction. J Vasc Surg. 2008;48(6):1451-7. DOI: 10.1016/j.jvs.2008.07.004 [ Links ]
Received: May 20, 2023; Accepted: June 15, 2023











 texto em
texto em 


