Meu SciELO
Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Angiología
versão On-line ISSN 1695-2987versão impressa ISSN 0003-3170
Angiología vol.75 no.6 Madrid Nov./Dez. 2023 Epub 29-Jan-2024
https://dx.doi.org/10.20960/angiologia.00542
Originals
Prospective randomized clinical trial on the impact of remote ischemic preconditioning on contrast induced nephropathy in patients treated endovascular aortic repair
2Hospital Clínico Universitario de Valladolid. Valladolid, Spain
Introduction:
the biggest drawback of using iodinated contrasts in clinical practice is contrast nephropathy, which increases morbidity and mortality and hospital costs. Remote ischemic preconditioning (RIPC) is a non-invasive tissue protection technique that has proven to be able to reduce renal involvement after intravascular contrast administration.
Objective:
the main goal of this study was to assess the impact of RIPC on the incidence of contrast-induced nephropathy in patients undergoing endovascular aortic repair (EVAR).
Material and methods:
patients who underwent elective EVAR were included, and then sequentially assigned to the control and preconditioning groups (groups C and p, respectively). Pre- and postoperative hematocrit (at 24, 72 hours, and 30 days) was analyzed.
Results:
a total of 98.3 % of the patients included in the study were men out of a total sample of 120 patients. The mean age was 73 years (range, 56-87). Diabetes and preoperative chronic kidney disease (understood as glomerular filtration rates < 60 mL/min) were present in 29.16 % and 38.33 % of the patients, respectively. Half of the sample received preconditioning in the preoperative period. A total of 24.17 % of the patients developed nephropathy despite fluid therapy with or without preconditioning. At the postoperative period (24 h-72 h), preconditioning did not modify the incidence rate of nephropathy, serum creatinine and urea, or even the estimated glomerular filtration rate (eGFR). However, at the 30-day follow-up the preconditioned group showed a significant improvement in serum creatinine and urea levels (1.46 ± 0.3 vs 1.03 ± 0.5; p < 0.001; 61.06 ± 27.5 mg/dL vs 43 .78 ± 12.9 mg/dL; p = 0.003) and eGFR increase (56.37 ± 23.4 mL/min/1.73 m2 vs 72.85 ± 17.7mL/min/1.73 m2; p = 0.004).
Conclusions:
RIPC seems effective in alleviating the effects of iodinated contrast on the kidneys of patients undergoing EVAR in the short term follow up (30 days).
Keywords EVAR; Remote ischemic preconditioning; Contrast induced nephropathy; Intravascular contrast media; Ischemia-reperfusion injury
INTRODUCTION
The main drawback associated with the use of iodinated contrast agents in the routine clinical practice is contrast-induced nephropathy (CIN), which increases morbidity, mortality, and hospital spending. Worsening renal function has been reported in 18 % to 23 % of the patients undergoing endovascular aneurysm repair (EVAR) in the postoperative period (1). CIN is defined as an acute deterioration of renal function between day 2 and day 7 after the administration of iodinated contrast, which should be > 0.5 mg/dL; 44 µmol/L in absolute terms or > 25 % in relative terms (2-5). The pathophysiology of CIN results from dysregulation between vasoconstriction and vasodilation of the renal parenchyma medullary vessels added to the direct cytotoxic effect of the contrast agent (6,7).
In addition to the already known modifiable and non-modifiable risk factors (8) associated with the development of CIN, patients undergoing EVAR have additional lesional mechanisms, such as microembolisms of the renal artery during device deployment (9), dissection of the renal artery ostium, ischemia-reperfusion syndrome (10), hypovolemia, and other cardiovascular risk factors (7,11).
It is recommended to assess the risk of developing CIN, minimize contrast volume and nephrotoxic drugs as much as possible, and follow a hydration protocol of 1 mL/kg/h to 1.5 mL/kg/h of a normal saline solution at 0.9 % for, at least, 12 hours up to 24 hours after contrast exposure (3,6,12,13). However, there are other nephroprotection strategies available, such as remote ischemic preconditioning (RIPC) (14,15).
RIPC is a non-invasive tissue protection technique that has been shown to reduce renal damage following the administration of contrast agents. Its mechanism of action is unknown (1), although some hypotheses suggest the involvement of complex humoral, neural, systemic, and molecular mechanisms (16) that result in anti-inflammatory effects and reduced extravascular levels of free radicals and lactate (17).
The objective of this study is to quantify the actual incidence of CIN and study the role of RIPC as a strategy to reduce the incidence of CIN in patients treated with elective EVAR.
MATERIAL AND METHODS
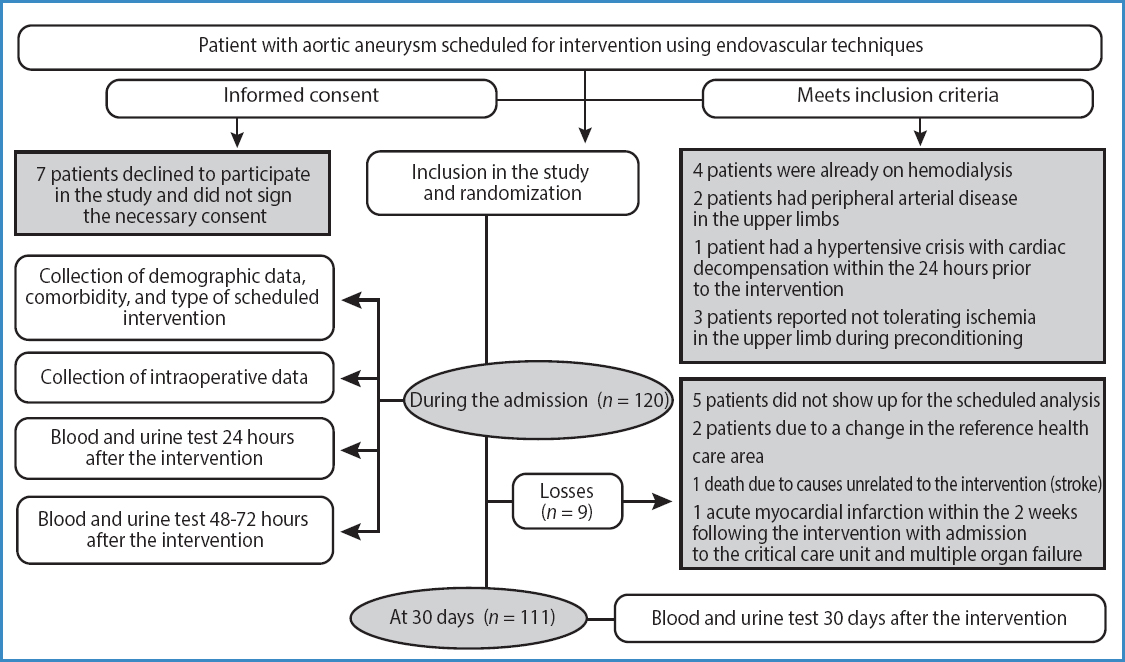
This was a prospective, randomized, clinical trial of patients undergoing elective-only EVAR from January 2017 through January 2019 at Hospital Clínico Universitario de Valladolid, Spain. This study did not receive any funding from the industry whatsoever and was conducted following the principles established by the Declaration of Helsinki. The research protocol was approved by the Clinical Research Ethics Committee of the Eastern Area of Valladolid (PI-20-1745). This study is registered on ClinicalTrials.gov with reference no. NCT05350683. Participation in this study was voluntary, and all participants gave their prior written informed consent.
Exclusion criteria were applied to patients with peripheral arterial disease in the upper limbs, on dialysis, with decompensated heart failure at the time of the intervention, or who could not tolerate the preconditioning protocol.
The study variables can be seen in table I. The impact of RIPC was measured in absolute and relative terms of creatinine variation within the first 48 hours after contrast administration and based on the aforementioned definition of CIN.
Table I. Sample description
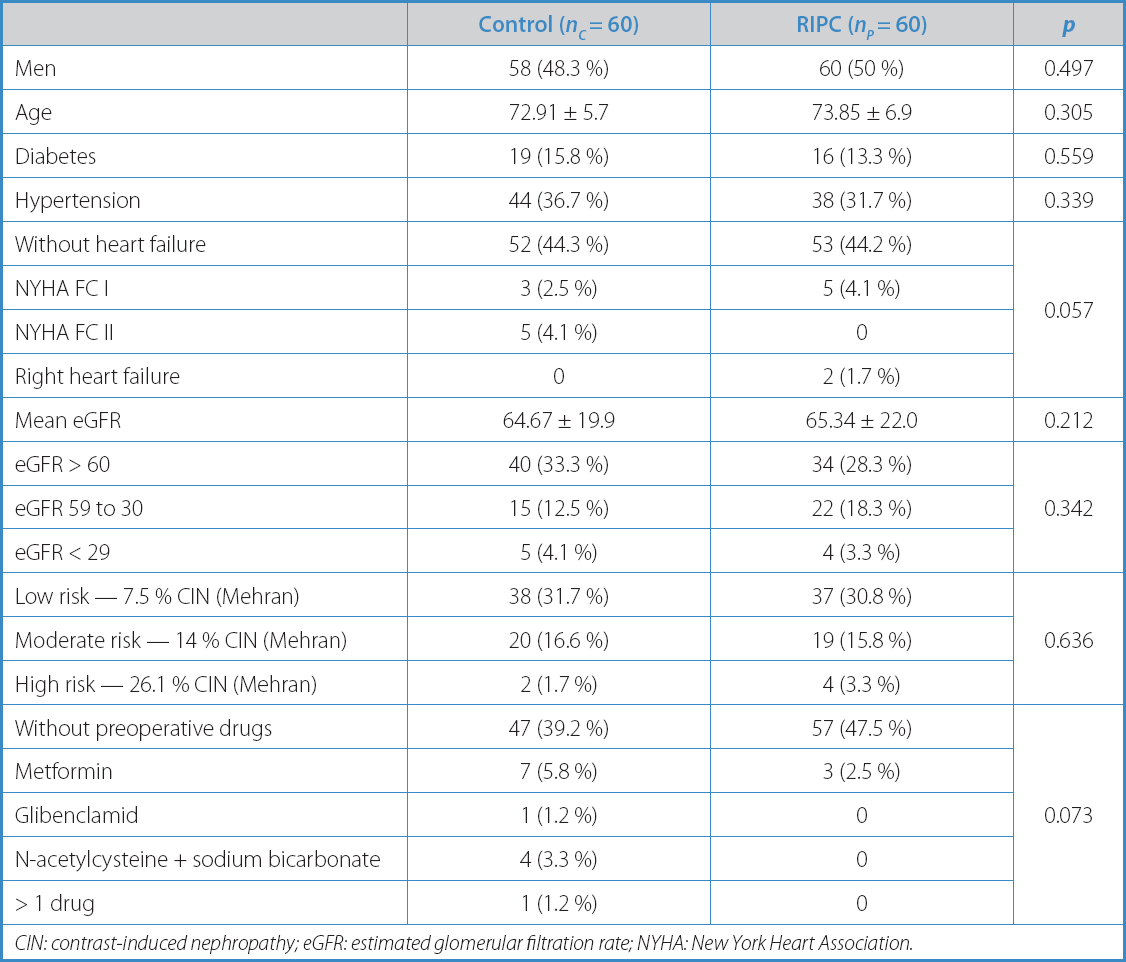
CIN: contrast-induced nephropathy; eGFR: estimated glomerular filtration rate; NYHA: New York Heart Association.
All patients received hydration therapy 12 hours before contrast administration, at a rate of 1 mL/kg/h, regardless of the risk of developing CIN (according to the Mehran scale [18]). Nephrotoxic drugs were discontinued 24 hours before surgery, and no other nephroprotection drug was used unless indicated by a specialist.
In the 12 hours prior to surgery, the lead investigator performed RIPC according to a standardized protocol by alternating 4 cycles of 5 min of cuff inflation on the non-dominant upper limb until SBP + 20 mmHg followed by 5 min of deflation. Patients were consecutively recruited on a 1:1 ratio.
The objective of this study is to quantify the actual incidence of CIN in patients undergoing elective EVAR and evaluate the role of RIPC as nephroprotection strategy in these patients.
The sample size was calculated using the GRANMO 5.2 calculator based on a mean difference in postoperative creatinine values of 0.7 mg/dL between the control and preconditioned groups (C and p, respectively) in a population similar to that of this study (17), with an 80 % statistical power and a bilateral type 1 error of 5 %. A preliminary calculation prior to recruitment resulted in 37 patients in each study arm. When 18 patients were recruited, the calculation was repeated, resulting in 65 patients in each study arm. A 10 % loss to follow-up was estimated.
Continuous variables were tested for normal distribution using the Shapiro-Wilk test. Normally distributed variables are expressed as mean and standard deviation and compared using independent t-tests and ANOVA for continuous variables with repeated measures. Non-normally distributed variables were analyzed using the Mann-Whitney U test. The categorical variables are expressed as frequency and percentage and compared using Pearson's chi-squared and Fisher's exact tests.
p values < 0.05 were considered statistically significant. All analyses were performed using SPSS, version 27.0; IBM Corporation, Somers, NY, United States.
RESULTS
A total of 120 patients were included (see flowchart in figure 1) with aortic aneurysmal disease treated with elective EVAR: 58 men and 2 women in group C, 60 men in group p; mean age of 72.91 ± 5.7 years (range, 60-83) in group C, and 73.85 ± 6.9 (range, 56-87) in group p.
Table I illustrates the descriptive analysis of the variables included based on the study groups. The surgical characteristics are shown in table II, and the postoperative ones in table III. The only differences seen between both groups were a higher incidence of NYHA FC II in group C (5 [4.1 %] vs 0; p = 0.057), and the use of metformin (7 [5.8 %] vs 3 [2.5 %]; p = 0.073) (Table I). Baseline renal function in terms of serum creatinine and serum urea levels, and the estimated glomerular filtration rate were similar in both groups (Table IV).
Table II. Surgical characteristics
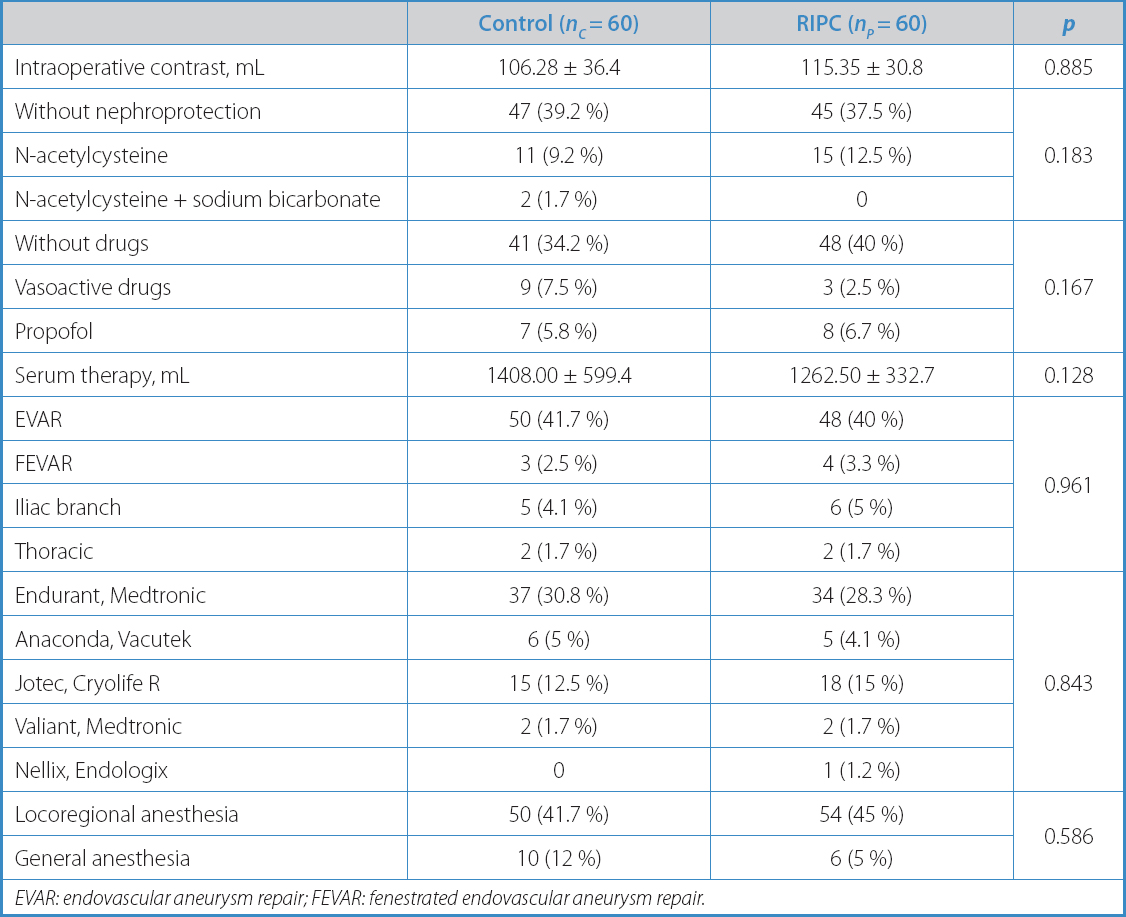
EVAR: endovascular aneurysm repair; FEVAR: fenestrated endovascular aneurysm repair.
Table IV. Continuous variables both pre- and postoperative
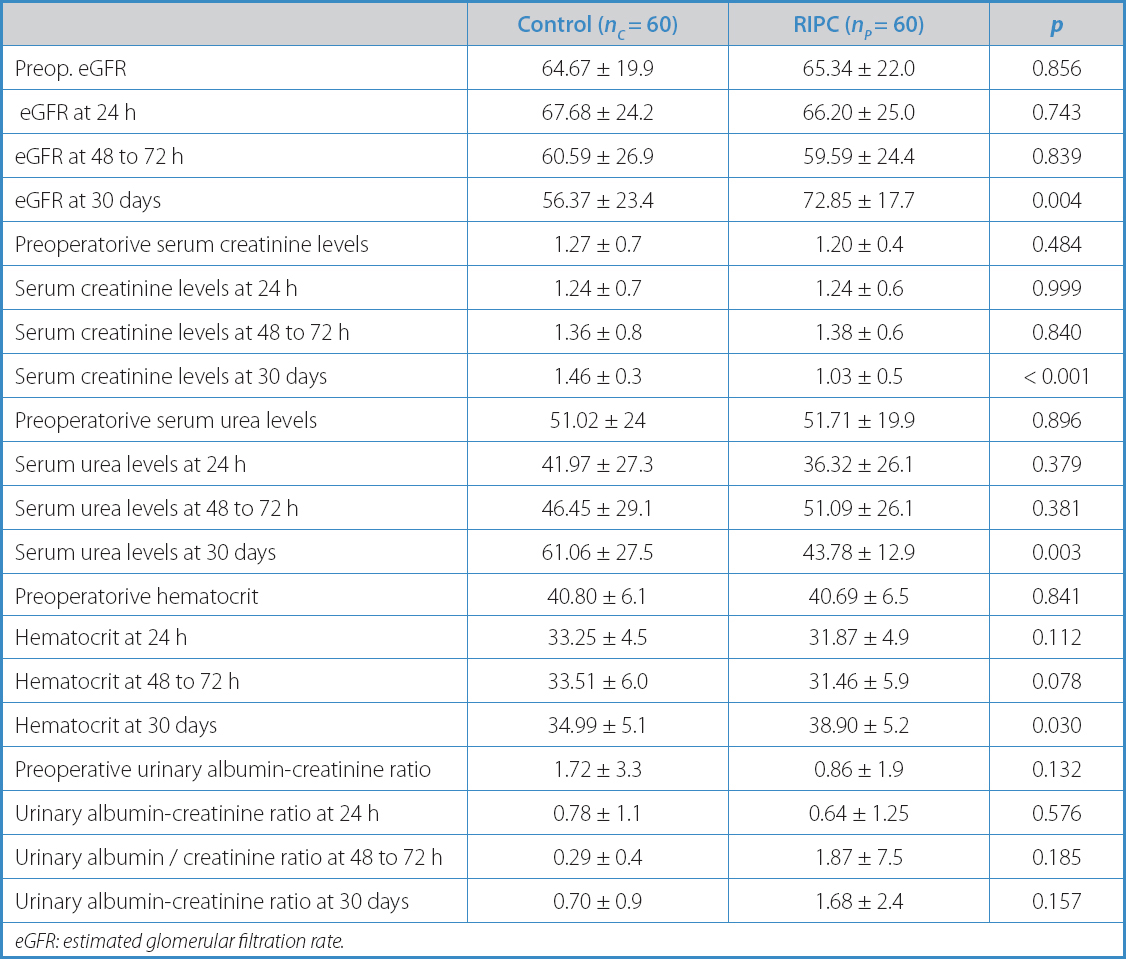
eGFR: estimated glomerular filtration rate.
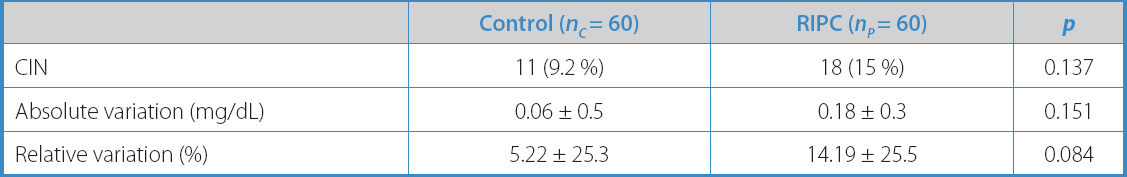
The variables describing the study primary endpoint are summarized in table V. There were no statistically significant differences between group p and group C in terms of relative (5.22 % ± 25.3 vs 14.19 % ± 25.5; p = 0.084) or absolute variation (0.06 mg/dL ± 0.5 vs 0.18 mg/dL ± 0.3; p = 0.151) in serum creatinine levels and, therefore, in the incidence of CIN (11 patients [9.2 %] vs 18 patients [15 %]; p = 0.137), considering the previously described definition of CIN.
Intraoperative variables, including the volume of contrast administered to the patients (106.28 ± 36.4 vs 115.35 ± 30.8; p = 0.885), did not differ significantly between the groups (Table II). Postoperative values are summarized in table III.
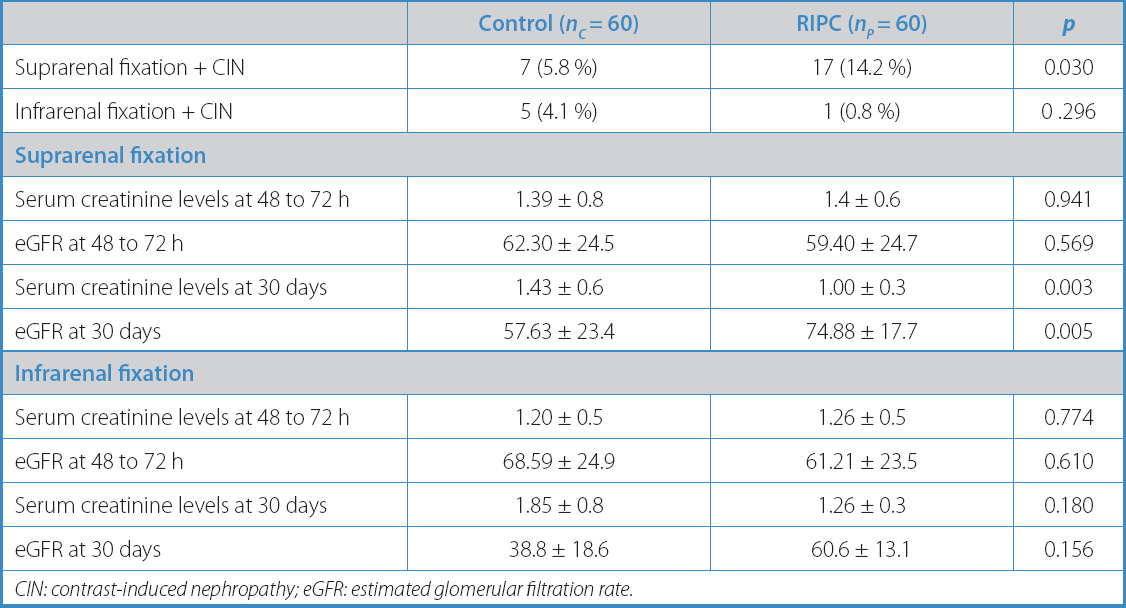
However, significant differences were found in serum creatinine and serum urea levels (1.46 ± 0.3 vs 1.03 ± 0.5; p < 0.001; 61.06 ± 27.5 vs 43.78 ± 12.9; p = 0.003), estimated glomerular filtration rate (56.37 ± 23.4 vs 72.85 ± 17.7; p = 0.004), and hematocrit (34.99 ± 5.1 vs 38.90 ± 5.2; p = 0.030) at 30 days in favor of the preconditioned group, with no statistical variation between the groups during the immediate postoperative period (24 h to 48 h) (Table IV).
In a subgroup analysis, only high-risk patients (Mehran marker > 11) who developed CIN during the immediate postoperative were compared. Four patients with CIN were obtained, all of whom were randomized to the preconditioned group. None of the 2 high-risk patients randomized to the control group experienced CIN (p = 0.014).
Another subgroup was created based on the type of stent-graft fixation used. In a first analysis, stent-grafts were arranged according to the name of the commercial brand used, and no significant differences were seen between the study arms (p = 0.843). However, stent-grafts of supra-renal fixation were much more common in both groups (104 vs 12; p = 0.034). Thoracic stent-grafts were excluded from this subanalysis. Regarding renal function, this new analysis showed no significant differences between the study arms regarding the serum creatinine levels or eGFR at 48 hours. On the other hand, there was a significant reduction in the serum creatinine levels and an improved eGFR in preconditioned patients with supra-renal fixation compared to those from the control arm at 30 days (Table VI).
DISCUSSION
The aim of this study was to assess the impact of RIPC on the incidence of CIN in patients undergoing elective EVAR. In this case, the incidence of CIN varied from group C to group p, although this difference was not clinically significant.
Possibly, the higher incidence rates of nephropathy reported in group p may be due to the presence of more patients being categorized as high-risk according to the Mehran scale (18). Since both study arms were initially comparable (Table I), the analysis of the incidence of nephropathy by risk subgroups only revealed the presence of 6 patients with Mehran scores > 11. It is possible that such a small number resulted in suboptimal randomization.
The actual incidence of CIN in patients undergoing elective EVAR surgery is unknown to this date because there is not such a thing as a widely accepted definition consistently used in the scientific medical literature available (1). Therefore, the documented incidence rate of CIN varies from 8.5 % to 28 %, depending on the series studied (1,11,19).
A search on PubMed using the terms “endovascular aneurysm repair” (MeSH terms) and “ischemic preconditioning” (MeSH terms) resulted in an article published by Walsh et al. in 2009 (14) that specifically included the target population of our study. A recent meta-analysis conducted by Stather et al. (15) was also found, which included all aortic surgeries performed, not only patients treated with EVAR.
The preconditioning protocol in this study used the non-dominant upper limb because the risk of peripheral arterial disease is lower compared to the lower limbs, and involved 4 cycles of 5 min of ischemia applied within the 12 hours prior to surgery. A study protocol published by Healy in 2015 (20) also used the upper limb, even though the skeletal muscle mass of the lower limb is greater than that of the arm. The upper limb has been more widely used for preconditioning due to its potential resistance to RIPC in diabetic and elderly patients (20-22).
Traditionally, the only proven nephroprotection strategy is fluid therapy (23), which is considered mandatory for patients with estimated glomerular filtration rates < 60 mL/min/1.73 m2 (24,25). In this study, fluid therapy was used in both groups to avoid biases resulting from its well-known nephroprotective effect. A review published in 2016 by Koch (26) shows that all studies used hyperhydration protocols before and after contrast infusion using 0.9 % sodium chloride aqueous solutions at infusion rates of 1 mL/kg/h to 4 mL/kg/h with different durations.
In this study, the renal function biomarkers used were serum and urinary creatinine, the estimated glomerular filtration rate, and the urinary albumin-creatinine ratio. Recently, numerous renal function biomarkers have been described, and attempts have been made to add them to the routine clinical practice (27). Despite its drawbacks (8), serum creatinine remains a universally used biomarker for renal function (28).
After a 30-day follow-up, the preconditioned group showed significantly lower serum creatinine and urea levels, along with an increased eGFR. Most scientific medical literature on RIPC stresses its potential as an immediate measure of renal protection against kidney damage, which is why the quantification of renal biomarkers is often stopped at 48-72 hours (29). Other studies extend follow-up, counting major adverse cardiovascular events up to 12 months (20,21), but they do not focus on renal impairment. Interestingly, the review published by Koch (26) reports a significant reduction of morbidity and mortality rates at 6 weeks, including death, rehospitalization, and the need for dialysis. The prolonged effect of RIPC may be justified by epigenetic (30) and chromosomal conformational changes (31), as well as molecular (27,30) and histological processes in the kidney that trigger tissue protection/repair mechanisms aimed at reducing the inflammatory reaction due to ischemia-reperfusion.
CIN, along with atheroembolic disease has a worse prognosis (11,32). The renal function impairment seen at the 30-day follow-up in group C from this study could be the result of CIN and microembolisms. In group p, a significantly positive evolution of creatinine, urea, and renal function (33) was observed.
The suprarenal fixation of the stent-graft (34), repeated contrast administration at the follow-up (35,36), or even surgical time (19) can lead to the occurrence of microembolisms and long-term worsening of renal function after EVAR. In this study, a subgroup analysis based on stent-graft fixation proved that RIPC may be particularly useful in cases of suprarenal fixation, where renal function is mainly impaired in the long term.
In this study, a drop in hematocrit of 7.55 % ± 1.6 % in the control group and 18.82 % ± 1.6 % in the preconditioning group was reported within the first 24 hours after surgery. Postoperative anemia after EVAR is often due to the catheters and introducer sheaths exchanged during surgery (37). In our study, the recovery of hematocrit in the preconditioning group was greater at the 30-day mark.
Experimental studies have demonstrated that even with reduced hematocrit levels, erythropoietin levels in a kidney damaged by ischemia-reperfusion injuries remain low due to a lower sensitivity to circulating oxygen (38). The positive evolution of hematocrit levels in patients undergoing RIPC could be justified by the protective effect of preconditioning against ischemia-reperfusion injury, which may have mitigated the drop of erythropoietin synthesis, thus favoring the recovery of erythropoietin levels, and therefore, hematocrit in the long term. This finding is the basis of a hypothesis not yet described in the medical literature that could be the subject of future studies.
This study has several limitations. Firstly, preconditioning was performed within 12 hours prior to the administration of contrast. Experimental studies show that ischemic preconditioning induces molecular changes that have a protective effect on tissues, not only in the short but also in the long term (39). The results of serum creatinine levels in the postoperative period could have been different if preconditioning would had been performed > 12 hours prior to the administration of contrast. Secondly, the control and preconditioning populations did not show significant differences regarding the type of anesthesia used. However, certain anesthetics can dampen the effect of preconditioning. Unfortunately, studies on this topic do not specifically address CIN in patients undergoing EVAR, so the results may not necessarily be extrapolated (40-42). Finally, this study cannot demonstrate whether there are racial or gender differences because the predominant population included male Caucasian participants.
CONCLUSIONS
In this study, RIPC did not demonstrate a significant effect on the incidence of CIN during the early postoperative period in patients undergoing EVAR. However, the disease progression of the RIPC group was favorable in parameters such as serum creatinine, urea, eGFR, and hematocrit 30 days after surgery, even in cases with suprarenal fixation. This situates RIPC as a simple and widely available nephroprotection strategy for patients treated with EVAR.
BIBLIOGRAFÍA
1. Saratzis A, Melas N, Mahmood A, et al. Incidence of Acute Kidney Injury (AKI) after Endovascular Abdominal Aortic Aneurysm Repair (EVAR) and Impact on Outcome. Eur J Vasc Endovasc Surg 2015;49(5):534-40. DOI:10.1016/j.ejvs.2015.01.002 [ Links ]
2. Nicola R, Shaqdan KW, Aran K, et al. ContrastInduced Nephropathy:Identifying the Risks, Choosing the Right Agent, and Reviewing Effective Prevention and Management Methods. Curr Probl Diagn Radiol 2015;44(6):501-4. DOI:10.1067/j.cpradiol.2015.04.002 [ Links ]
3. Tsai TT, Patel UD, Chang TI, et al. Contemporary incidence, predictors, and outcomes of acute kidney injury in patients undergoing percutaneous coronary interventions:insights from the NCDR CathPCI registry. JACC Cardiovasc Interv 2014;7(1):1-9. DOI:10.1016/j.jcin.2013.06.016 [ Links ]
4. Azzalini L, Spagnoli V, Ly HQ. Contrast-Induced Nephropathy:From Pathophysiology to Preventive Strategies. Can J Cardiol 2016;32(2):247-55. DOI:10.1016/j.cjca.2015.05.013 [ Links ]
5. Owen RJ, Hiremath S, Myers A, et al. Canadian Association of Radiologists consensus guidelines for the prevention of contrastinduced nephropathy:up-date 2012. Can Assoc Radiol J 2014;65(2):96-105. DOI:10.1016/j.carj.2012.11.002 [ Links ]
6. Mehta RL, Kellum JA, Shah SV, et al;Acute Kidney Injury Network. Acute Kidney Injury Network:report of an initiative to improve outcomes in acute kidney injury. Crit Care 2007;11(2):R31. DOI:10.1186/cc5713 [ Links ]
7. Nash K, Hafeez A, Hou S. Hospital-acquired renal insufficiency. Am J Kidney Dis 2002;39(5):930-6. DOI:10.1053/ajkd.2002.32766 [ Links ]
8. Sociedad Española de Nefrología. Guías SEN:Actuación en el Fracaso Renal Agudo 2007;27(Suppl.3):1-276. [ Links ]
9. Seeliger E, Sendeski M, Rihal CS, et al. Contrastinduced kidney injury:mechanisms, risk factors, and prevention. Eur Heart J 2012;33(16):2007-15. DOI:10.1093/eurheartj/ehr494 [ Links ]
10. Kubota H. Remote ischemic preconditioning:Simple method but unknown mechanisms. J Cardiol 2016;67(1):34-5. DOI:10.1016/j.jjcc.2015.07.006 [ Links ]
11. Gutiérrez Castillo D, San Norberto EM, Fidalgo Domingos L, et al. Incidência De Nefropatia Induzida Por Contraste Em Pacientes Operados De Reparação Aórtica Endovascular. Rev Port Cir Cardiotorac Vasc 2016;22(2):101-7. [ Links ]
12. Schilp J, de Blok C, Langelaan M, et al. Guideline adherence for identification and hydration of highrisk hospital patients for contrastinduced nephropathy. BMC Nephrol 2014;15:2. DOI:10.1186/1471-2369-15-2 [ Links ]
13. Soni SS, Ronco C, Katz N, et al. Early diagnosis of acute kidney injury:the promise of novel biomarkers. Blood Purif 2009;28(3):165-74. DOI:10.1159/000227785 [ Links ]
14. Walsh SR, Boyle JR, Tang TY, et al. Remote ischemic preconditioning for renal and cardiac protection during endovascular aneurysm repair:a randomized controlled trial. J Endovasc Ther 2009;16(6):680-9. DOI:10.1583/09-2817.1 [ Links ]
15. Stather PW, Wych J, Boyle JR. A systematic review and metaanalysis of remote ischemic preconditioning for vascular surgery. J Vasc Surg 2019;70(4):1353-63.e3. DOI:10.1016/j.jvs.2019.03.025 [ Links ]
16. McDonald RJ, McDonald JS, Bida JP, et al. Intravenous contrast materialinduced nephropathy:causal or coincident phenomenon? Radiology 2013;267(1):106-18. DOI:10.1148/radiol.12121823. Erratum in:Radiology 2016;278(1):306. [ Links ]
17. Er F, Nia AM, Dopp H, et al. Ischemic preconditioning for prevention of contrast mediuminduced nephropathy:randomized pilot RenPro Trial (Renal Protection Trial). Circulation 2012;126(3):296-303. DOI:10.1161/CIRCULATIONAHA.112.096370 [ Links ]
18. Mehran R, Aymong ED, Nikolsky E, et al. A simple risk score for prediction of contrastinduced nephropathy after percutaneous coronary intervention:development and initial validation. J Am Coll Cardiol 2004;44(7):1393-9. DOI:10.1016/j.jacc.2004.06.068 [ Links ]
19. Sailer AM, Nelemans PJ, van Berlo C, et al. Endovascular treatment of complex aortic aneurysms:prevalence of acute kidney injury and effect on longterm renal function. Eur Radiol 2016;26(6):1613-9. DOI:10.1007/s00330-015-3993-8 [ Links ]
20. Healy D, Clarke-Moloney M, Gaughan B, et al. Preconditioning Shields Against Vascular Events in Surgery (SAVES), a multicentre feasibility trial of preconditioning against adverse events in major vascular surgery:study protocol for a randomised control trial. Trials 2015;16:185. DOI:10.1186/s13063-015-0678-1 [ Links ]
21. Mouton R, Pollock J, Soar J, et al. Remote ischaemic preconditioning versus sham procedure for abdominal aortic aneurysm repair:an external feasibility randomized controlled trial. Trials 2015;16:377. DOI:10.1186/s13063-015-0899-3 [ Links ]
22. Thomas KN, Cotter JD, Williams MJ, et al. Repeated Episodes of Remote Ischemic Preconditioning for the Prevention of Myocardial Injury in Vascular Surgery. Vasc Endovascular Surg 2016;50(3):140-6. DOI:10.1177/1538574416639150 [ Links ]
23. Sadat U, Walsh SR, Norden AG, et al. Does oral N-acetylcysteine reduce contrast-induced renal injury in patients with peripheral arterial disease undergoing peripheral angiography?A randomizedcontrolled study. Angiology 2011;62(3):225-30. DOI:10.1177/0003319710377078 [ Links ]
24. Fox AJ, Symons SP, Aviv RI. Contrastinduced nephropathy and dehydration. Can Assoc Radiol J 2013;64(3):275. DOI:10.1016/j.carj.2012.12.004 [ Links ]
25. Gupta RK, Bang TJ. Prevention of Contrast-Induced Nephropathy (CIN) in Interventional Radiology Practice. Semin Intervent Radiol 2010;27(4):348-59. DOI:10.1055/s-0030-1267860 [ Links ]
26. Koch C, Chaudru S, Lederlin M, et al. Remote Ischemic Preconditioning and Contrast-Induced Nephropathy:A Systematic Review. Ann Vasc Surg 2016;32:176-87. DOI:10.1016/j.avsg.2015.10.017 [ Links ]
27. Andreucci M, Faga T, Pisani A, et al. The ischemic/nephrotoxic acute kidney injury and the use of renal biomarkers in clinical practice. Eur J Intern Med 2017;39:1-8. DOI:10.1016/j.ejim.2016.12.001 [ Links ]
28. D'Amore C, Nuzzo S, Briguori C. Biomarkers of Contrast-Induced Nephropathy:Which Ones are Clinically Important?Interv Cardiol Clin 2020;9(3):335-44. DOI:10.1016/j.iccl.2020.02.004 [ Links ]
29. García S, Rector TS, Zakharova M, et al. Cardiac Remote Ischemic Preconditioning Prior to Elective Vascular Surgery (CRIPES):A Prospective, Randomized, Sham-Controlled Phase II Clinical Trial. J Am Heart Assoc 2016;5(10):e003916. DOI:10.1161/JAHA.116.003916 [ Links ]
30. Park KM, Byun JY, Kramers C, et al. Inducible nitricoxide synthase is an important contributor to prolonged protective effects of ischemic preconditioning in the mouse kidney. J Biol Chem 2003;278(29):27256-66. DOI:10.1074/jbc.M301778200 [ Links ]
31. Heyman SN, Leibowitz D, Mor-Yosef Levi I, et al. Adaptive response to hypoxia and remote ischaemia preconditioning:a new hypoxiainducible factors era in clinical medicine. Acta Physiol (Oxf) 2016;216(4):395-406. DOI:10.1111/apha.12613 [ Links ]
32. Sociedad Española de Nefrología. Documento de la Sociedad Española de Nefrología sobre las guías KDIGO para la evaluación y el tratamiento de la enfermedad renal crónica. Nefrología 2014;34:302-16. [ Links ]
33. Bersani-Amado LE, da Rocha BA, Schneider LCL, et al. Nephropathy induced by renal microembolism:a characterization of biochemical and histopathological changes in rats. Int J Clin Exp Pathol 2019;12(6):2311-23. [ Links ]
34. Stather PW, Ferguson J, Awopetu A, et al. Metaanalysis of Renal Function Following Infrarenal EVAR using Suprarenal or Infrarenal Fixation Devices. Eur J Vasc Endovasc Surg 2018;56(4):486-96. DOI:10.1016/j.ejvs.2018.01.021 [ Links ]
35. Walsh SR, Tang TY, Boyle JR. Renal consequences of endovascular abdominal aortic aneurysm repair. J Endovasc Ther 2008;15(1):73-82. DOI:10.1583/07-2299.1 [ Links ]
36. Twine CP, Boyle JR. Renal dysfunction after EVAR:time for a standard definition. J Endovasc Ther 2013;20(3):331-3. DOI:10.1583/12-4104C.1 [ Links ]
37. Sen I, Tenorio ER, Mirza AK, et al. Effect of Blood Loss and Transfusion Requirements on Clinical Outcomes of Fenestrated-Branched Endovascular Aortic Repair. Cardiovasc Intervent Radiol 2020;43(11):1600-7. DOI:10.1007/s00270-020-02573-8 [ Links ]
38. Tan CC, Tan LH, Eckardt KU. Erythropoietin production in rats with postischemic acute renal failure. Kidney Int 1996;50(6):1958-64. DOI:10.1038/ki.1996.518 [ Links ]
39. Zarbock A, Kellum JA, Van Aken H, et al. Longterm Effects of Remote Ischemic Preconditioning on Kidney Function in Highrisk Cardiac Surgery Patients:Follow-up Results from the RenalRIP Trial. Anesthesiology 2017;126(5):787-98. DOI:10.1097/ALN.0000000000001598 [ Links ]
40. Cho YJ, Nam K, Kim TK, et al. Sevoflurane, Propofol and Carvedilol Block Myocardial Protection by Limb Remote Ischemic Preconditioning. Int J Mol Sci 2019;20(2):269. DOI:10.3390/ijms20020269 [ Links ]
41. Benstoem C, Goetzenich A, Stoppe C. The role of propofol for remote ischaemic preconditioning in the setting of cardiac surgery - a Cochrane systematic review. Br J Anaesth 2017;119(6):1234-5. DOI:10.1093/bja/aex357 [ Links ]
42. Cheung CX, Healy DA, Walsh SR. Remote preconditioning and cardiac surgery:regrouping after Remote Ischemic Preconditioning for Heart Surgery (RIPHeart) and Effect of Remote Ischemic Preconditioning on Clinical Outcomes in Patients Undergoing Coronary Artery Bypass Surgery (ERICCA). J Thorac Dis 2016;8(3):E197-9. DOI:10.21037/jtd.2016.01.81 [ Links ]
Received: June 17, 2023; Accepted: August 26, 2023











 texto em
texto em