Meu SciELO
Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Nutrición Hospitalaria
versão On-line ISSN 1699-5198versão impressa ISSN 0212-1611
Nutr. Hosp. vol.27 no.2 Madrid Mar./Abr. 2012
Palm tree syrup; nutritional composition of a natural edulcorant
Miel de palma; composición nutricional de un edulcorante natural
G. Luis1, C. Rubio1, A. J. Gutiérrez1, C. Hernández1, D. González-Weller2, C. Revert1, A. Castilla1, P. Abreu3 and A. Hardisson1
1Department of Toxicology. University of La Laguna. Tenerife. Canary Island. Spain.
2Canary Health Service. S/C de Tenerife. Spain.
3Department of Physiology. University of La Laguna. Tenerife. Canary Island. Spain.
ABSTRACT
Introduction: Palm syrup is a typical product from the Canary Islands, traditionally produced from the sap of the tropical palm tree Phoenix canariensis. Its high caloric content has led to its increasing use as a health food supplement for athletes, children and elderly. Furthermore, demand for this natural syrup is continuously increasing due also to its medicinal uses in homeopathic medicine.
Objective: Palm Tree syrup samples prepared with palm sap from primary producers in La Gomera island (Canary Islands, Spain) were analyzed for their nutritional composition (moisture, ash, sugars, fat, vitamins and minerals).
Methods: 35 syrup samples from five different producing regions in La Gomera island were analyzed. High-performance liquid chromatography (HPLC) was used to determine sugars and vitamins and Flame Atomic Absorption Spectrophotometry (FAAS) was used to analyze the minerals.
Results: Major carbohydrates were sucrose (37.8%), glucose (9.50%) and fructose (4.80%), respectively. The presence of arabinose could not be confirmed. Niacin was the water-soluble vitamin with the highest concentration with an average content of 0.003%. Fat content was found to be under 0.20%. Potassium was the mineral with highest contents (0.45%).
Conclusions: Results suggest that palm tree syrup can play an important role as a sugar and mineral source in human nutrition, suggesting that future applications for this product could be developed.
Key words: Syrup. Sucrose. Vitamins. Minerals. Highperformance liquid chromatography (HPLC).
RESUMEN
Introducción: La miel de palma es un producto típico de las Islas Canarias, tradicionalmente producido a partir de la savia de la palmera Phoenix canariensis. El alto contenido calórico lo convierte en un producto cada vez más utilizado como suplemento alimenticio para atletas, niños y mayores. Además, la demanda de este sirope natural, está en constante crecimiento debido también a sus usos medicinales en la medicina homeopática.
Objetivo: Analizar la composición nutricional (humedad, cenizas, azúcares, grasa, vitaminas y minerales) de las muestras de miel de palma preparadas con la savia de las palmeras de los productores de la isla de La Gomera (Islas Canarias, España).
Métodos: 35 muestras de miel de palma procedentes de cinco regiones productoras de la isla de La Gomera fueron analizadas. Los azúcares y las vitaminas fueron medidos mediante cromatografía líquida de alta resolución (CLAR) y la espectrofotometría de absorción atómica por llama (FAAS) se usó para determinar los minerales.
Resultados: Los hidratos de carbono mayoritarios fueron sacarosa (37,8%), glucosa (9,50%) y fructosa (4,80%), respectivamente. La presencia de arabinosa no pudo ser confirmada. La niacina es la vitamina hidrosoluble con la mayor concentración con un contenido promedio de 0,003%. Los contenidos en grasas se encontraron por debajo de 0,20%. El potasio era el macroelemento con los contenidos más altos (0,45%).
Conclusiones: Los resultados sugieren que la miel de palma puede jugar un papel importante como una fuente de azúcar y minerales en la nutrición humana y se pueden desarrollar futuras aplicaciones para este producto.
Palabras clave: Sirope. Sacarosa. Vitaminas. Minerales. Cromatografía líquida de alta resolución (CLAR).
Abbreviations
FAAS: Flame Atomic Absorption Spectrometry.
HPLC: High-performance liquid chromatography.
HNO3: Nitric acid.
H2SO4: Sulphuric acid.
b.p.: Bring to boiling.
UV detection: Ultraviolet detection.
SRM: Standard Reference Material.
NIST: National Institute of Standard and Technology.
Introduction
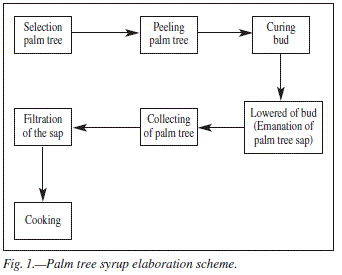
Palm syrup is a typical product from the Canary Islands, described as having been prepared by the former inhabitants of the islands before the arrival of the Spaniards.1 Palm syrup has been traditionally produced from the sap of the tropical palm tree Phoenix canariensis.2-3 For that reason, the palm tree sap, known as "guarapo", is collected from the palm trees in the early spring, when temperatures fluctuate between -5 and -10 oC. Then it must be boiled in a large pot on a wood fired stove until it becomes concentrated.4-5 The unique flavour of this natural product develops during this evaporation process (93-110 oC for 1.5 h). The flow-sheet to obtain the syrup is shown in figure 1, beginning with the extraction of palm tree sap6.
The resulting syrup is a viscous, sweet liquid, dark with reddish tones. Although this natural product has a similar density to honey, it is less sticky. The composition of the final product depends on the botanical origin, time and temperature of storage.7-9 Authors have reported physicochemical changes and degradation of the syrup due to overheating.10 For that reason, is important to ensure the stability of the syrup to protect against fermentation and crystallization.11-12
Although palm syrup is produced only in limited quantities, this natural product has a high commercial value because its unique flavour has made it popular in the confectionery industry and among consumers.1 Palm tree syrup is used by the Canarian population to flavour cakes, desserts, food coating or mixed drinks.13 For that reason, its high caloric content has led to its increasing use as a health food supplement for athletes, children and elderly.14 Furthermore, the demand for this natural syrup is continuously increasing due also to its medicinal uses in homeopathic medicine.
Palm tree syrup does not contain any kind of additive or artificial colourings and can last for years under the right storage conditions. Very few studies about the chemical and nutrient composition of palm tree syrup have been carried out. Nevertheless, producers and consumers are demanding more and more data about its composition to protect against adulteration via the addition of inexpensive sweeteners, and also for nutritional reasons. As the nutrient composition of Palm Tree syrup varies significantly depending on its geographical origin, it is possible to detect its adulteration and to ensure product purity.
The aim of this study was to determine the nutrient composition of palm tree syrup.
Materials and methods
Samples collection
A total of 35 samples of syrup were studied. Samples were collected during a sixteen month production period, between March 2007 and July 2008, and were analyzed. All samples were provided by producers from five different regions (Alojera, Taguluche, Tazo, Valle Gran Rey, Vallehermoso) of La Gomera island (Canary Islands, Spain). Random samples were collected in a manner proportional to the quantities obtained in each producing area. The sampling carried out in all zones was homogeneous. All samples were stored at -18 oC before being analyzed. Approximately 100 g of each sample was divided into three sections: (1) 20 g were used to determine sugar and fat contents, (2) 50 g to determine metal content and (3) 30 g to determine vitamin content. All measurements were made in triplicate.
Nutrient analysis
Moisture content was determined by weighing the samples before and after being dried for 48 h at 85 oC, and ash content was determined by heating the samples for 48 h at 450 oC, according to the methods of the Association of Official Analytical Chemists.15
High-Performance Liquid Chromatography (HPLC) techniques are often employed for the analysis of carbohydrates.16
Sugars analysis was carried out by means of HPLC employing a Shimadzu chromatograph equipped with a RID-6A detector using an Aminex HPX-87C column (250 x 4 mm).17-18 The columns were first conditioned by passing 2 mL of methanol through them and then washed with 6 mL of watermill-Q. Columns were used with acetonitrile/water mixtures (80:20) as an eluent relying on the hydrophilic interaction of the sugars with a water layer solvating the column. In this case, glucose, fructose, and sucrose concentrations were determined by injecting 20 μL of the standard solutions or sample extracts and eluting them with ultra pure water at flow rate of 2 mL/min. For that reason, refractive index detection has been used with its attendant drawbacks of limited sensitivity and instability with regard to fluctuations in mobile phase composition and eluent temperature.
Carbohydrates were quantified by comparison to appropriate standards.
The extracted fat was determined by a gravimetric procedure. 1 g of palm syrup was accurately weighed in a fat extraction flask. Water was added to bring the volume to 10 mL and mixed. 10 mL of hydrochloric acid (HCl) were added slowly and the content was again well mixed. The fat extraction flask was set in a heated water bath at 70-80 oC, brought to a boil and heated again at boiling point for 30 min while the flask was shaken carefully every 5 min. The content was cooled to room temperature. 25 mL of ether was added to the flask and then it was shaken vigorously for 1 min. 25 mL of light petroleum ether was added and shaken again. The ether and light petroleum steps were each repeated twice more. Finally, the solvents were evaporated and the extracted fat dried to constant weight in an oven at 103 ± 2 oC for 1 h, cooled to room temperature in a desiccator and weighed.
Most vitamins were analyzed by HPLC, vitamin B9 (folate acid) by the microbiological method and vitamin C (ascorbic acid) by titration. Vitamin B1 (thiamine) was extracted using 0.1 N sulphuric acid. Quantification was done with reversed-phase HPLC and post-column derivatization with fluorescence detection. Riboflavin (vitamin B2) and its coenzyme forms were extracted with 0.1 N sulphuric acid in an autoclave at 120 oC. After neutralization, the enzyme treatment removed the phosphate groups. The riboflavin content in the extract was determined by reversed-phase HPLC and fluorescence detection. Vitamin B3 (nicotin acid) was determined by reversedphase HPLC and UV detection after extraction with 0.001 N sulphuric acid.
Vitamin B6 (piridoxine) was extracted using sodium acetate buffer and analyzed using reversed-phase HPLC with fluorescence detection. Vitamins B12 and B9 were analyzed after aqueous extraction by means of microbiological assays. Vitamin D3 (cholecalciferol) was extracted with hexane after alkaline saponification. D2 was used as internal standard. The crude extract was cleaned in a semi-preparative HPLC. The fraction containing D2 and D3 was quantified by HPLC with UV detection.
Vitamin E (α-tocopherol) was analyzed after alkaline saponification and extraction with hexane by HPLC and fluorescence detection. Vitamin K was determined following enzymatic digestion, extraction and subsequent HPLC with post-column reduction with zinc and fluorescence detection.15
Hydrosoluble vitamins were analyzed with a Shimizu LC 6A chromatograph equipped with a ODS column (4.0 x 250 mm, 0.5 µm). Elution was at the rate of 1 mL/min with 0.1 mol/L ammonium acetate-acetic acid (pH 4.5)/methanol (75:25) at 30 oC. Detection was performed with a variable-wavelength UV detector at 254 nm.
Syrup samples were placed in porcelain crucibles and dried in an oven at 450 oC for 48 h. All samples were then introduced into a muffle oven and burned to ash at 450 oC. The white ashes obtained with this procedure were then dissolved in 5 % nitric acid (HNO3) to a volume of 50 mL.19
Mineral contents were determined using a flame atomic absorption spectrophotometer (FAAS) model Varian Spectr AA-10 Plus equipped with a deuterium lamp for background correction and hollow-cathode lamps for each of the studied elements. Quantification was performed using external standards (Merck IV, multielement standard solution) and all the standard curves were obtained at 5 different concentrations. Table I shows the instrumental parameters for the determination of microelements and macroelements by FAAS.
Quality control
Quality control of the analytical measurements was performed using blank samples and the following reference material: SRM-1515 (Apple leaves) from the National Institute of Standard and Technology (NIST). The recoveries obtained with the reference material were all above 98%.
All glassware was washed with acid and rinsed with purified water.
Results and discussion
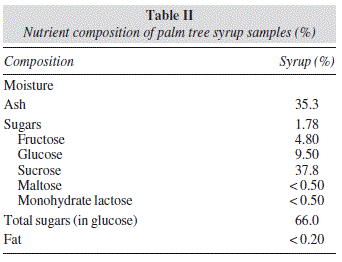
Table II shows the results of the moisture, ash, sugars and fat analysis.
Moisture content in this natural product was at 35.3% and the total solid at 64.7%. Water content can vary depending on the environmental conditions of the island.20-21
Ash content in palm syrup was 1.78%. Some studies have found that the ash content for honey in the province of Santa Cruz of Tenerife is 0.31%.22
All palm tree syrup samples contained significant amounts of sugars (66.0%). Sugars analysis indicated that all the palm tree syrup samples were rich in monosaccharides and disaccharides and that the sugars identified in the palm tree syrup samples with semi-quantitative values were sucrose, glucose and fructose. The levels of the three main sugars are quite similar in all samples despite geographical differences.23-24 The major carbohydrate found in the analyzed palm tree syrup samples was sucrose (37.8%) followed by glucose (9.50%) and fructose (4.80%), respectively.
Results published by other authors show that levels of sucrose (in the 60-83% range) are higher that the mean values found in this study and that levels of glucose (in the 3.15-5.04% range) are lower that our mean value (9.50%).1
All samples contained other disaccharides like maltose and lactose monohydrate at levels below 0.5%, but the presence of the monosaccharide arabinose could not be confirmed. Maltose and lactose monohydrate originate from enzymes by the formation of glycosidic bonds between monosaccharide, disaccharide and oligosaccharide. 25 Palm honey is considered to be a very rich source of myo-inositol (in the 3-8 mg/g range).1
Fats were found in very low proportions, with a content of less than 0.20%.
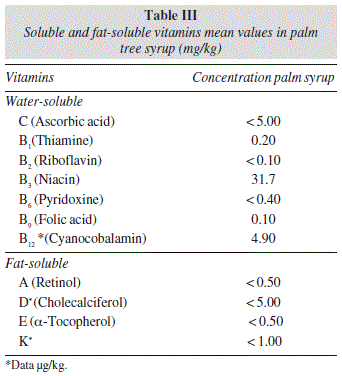
Content of water-soluble and fat-soluble vitamins in palm tree syrup samples are shown in table III. In general, the total vitamin content was low, below the level of nutritional importance. The water-soluble vitamin content is higher than that of fat-soluble vitamins. Regarding the fat-soluble vitamins, the palm tree syrup samples and the palm tree sap samples showed similar levels. Concentrations are arranged in the following sequence: A > E > D3 > K. Vitamins D (cholecalciferol) and K were rarely detectable, with concentrations less that 5.00 μg/kg and 1.00 μg/kg, respectively.
In all palm tree syrup samples, the water-soluble vitamin niacin showed the highest concentration (31.7 mg/kg) followed by ascorbic acid (5.00 mg/kg), cyanobalamin (5.00 mg/kg), pyridoxine (0.40 mg/kg), thiamine (0.20 mg/kg) riboflavin (0.10 mg/kg) and folic acid (0.10 mg/kg).
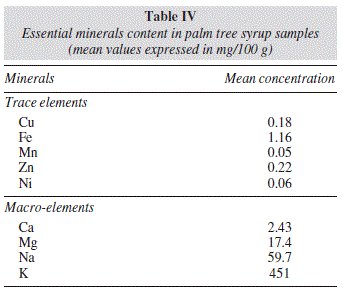
Table IV reflects the results for the average concentrations of the macro elements and trace elements. In all samples that were studied, potassium was the macro-element with the highest concentration (451 mg/100 g fresh sample),26-27 followed by sodium (59.7 mg/100 g fresh sample). As for micro-elements, iron presented the highest levels (1.16 mg/100 g) and manganese presented the lowest levels (0.05 mg/100 g).
Conclusions
Palm tree syrup is a supersaturated solution of sugars and water, suggesting that this natural product could be a suitable raw material in the production of natural sweeteners.
The vitamin content of palm tree syrup is higher than that of other syrups of botanical origin.
Although the fat, vitamin and mineral contents of palm tree syrup are low, this natural product contains a considerable amount of potassium and sodium. Overall, the results of this study suggest that palm tree syrup can play an important role as a mineral source in human nutrition.
Further studies should investigate new applications for this syrup, like its use as a sweetener.
References
1. Ruíz-Matute AI, Soria AC, Sanz ML, Martínez-Castro I. Characterization of tradicional Spanish edible plant syrups base on carbohydrate GC-MS analysis. J Food Compos Anal 2010; 23 (3): 260-3. [ Links ]
2. Asociación Canaria para la Enseñanza de las Ciencias; 2006 (updated 2008 September 28; cited 2008 October 20). Available from http://www.vierayclavijo.org/ [ Links ]
3. Diccionario de historia natural de las Islas Canarias. 1st ed. Mancomunidad de Cabildos de Las Palmas: J. Viera-Clavijo; 2004. Nivaria; p. 365. [ Links ]
4. Asociación Insular de Desarrollo Rural de La Gomera; 2005 (updated 2008 September 2; cited 2008 September 16). Available from http://www.aidergomera.com. [ Links ]
5. AIDER La Gomera. La cultura de la Palma (I). La Gomera; c2004-2011 (updated 2008 September 2; cited 2008 September 14). Available from http://www.bienmesabe.org/ [ Links ]
6. Quintero A, Marín C. Miel y Palma. Consejería de Agricultura y Pesca. Gobierno Autónomo de Canarias. Santa Cruz de Tenerife: Algol; 1985, pp. 28-43. [ Links ]
7. Amin WA, Safwat M, Känzig A. Quality criteria of treacle (black honey). Food Chem 1999; 67: 17-20. [ Links ]
8. Anklam E. A review of the analytical methods to determine the geographical and botanical origin of honey. Food Chem 1998; 63: 549-62. [ Links ]
9. Lachman J, Kolihová D, Miholová D, Kosata J, Titera D, Kult K. Analysis of minority honey components: Possible use for the evaluation of honey quality. Food Chem 2007; 101: 973-9. [ Links ]
10. Nagai T, Inove R, Kanamori N, Suzuki N, Nogashima T. Characterization of honey from different floral sources. Its functional properties and effects of honey species on storage of meat. Food Chem 2006; 97: 256-62. [ Links ]
11. Azeredo LC, Azeredo MA, de Souza SR, Dutra VML. Protein contents and physicochemical properties in honey samples of Apis mellifera of different floral origins. Food Chem 2008; 80: 249-54. [ Links ]
12. Liviu M, Dezmirean D, Moise A, Bobis O, Laslo L, Bogdanov S. Physico-chemical and bioactive properties of different floral origin honeys from Romania. Food Chem 2009; 112: 863-7. [ Links ]
13. Padilla C. La miel de Palma. Tenerife: Narria Estudios de artes y costumbres populares, 1980. [ Links ]
14. Juszczak L, Socha R, Roznowski J, Fortuna T, Nalepka K. Physicochemycal properties and quality parameters of herbhoneys. Food Chem 2009; 113: 538-42. [ Links ]
15. Association of Official Analytical Chemists. 16th ed. Washington, 1995. [ Links ]
16. Míguez-Bernárdez M, de la Montaña-Miguélez J, García Queijeiro J. HPLC determination of sugars in varieties of chestnut fruits from Galicia (Spain). J Food Compos Anal 2004; 17: 63-7. [ Links ]
17. Swallow KW, Low NH. Analysis and quantification of the carbohidrates in honey using high-perfomance liquid chromatography. J Agric Food Chem 1999; 38: 1828-32. [ Links ]
18. Cotte JF, Casablanca H, Chardon S, Lheritier JL, Grenier MF. Chromatographic analysis of sugars applied to the characterization of monofloral honey. Anal Bioanal Chem 2004; 380: 698-705. [ Links ]
19. Frías I, Rubio C, González-Iglesias T, Gutiérrez AJ, González D, Hardisson A. Metals in fresh honeys from Tenerife Island, Spain. Bull Environ Contam Toxicol 2008; 80: 30-33. [ Links ]
20. Murat K, Sevgy K, Sengül K, Esra U, Cemalettin B, Ferda C. Biological activities and chemical composition of three honeys of different types from Anatolia. Food Chem 2007; 100: 526-34. [ Links ]
21. Sanjuan E, Estupiñán S, Millán R, Casteló M, Penedo JC. Cardona A. Contribution to the quality evaluation and the water activity prediction of La Palma Island honey. J Food Qual 1997; 20: 225-34. [ Links ]
22. Frías I, Hardisson A. Color y contenido mineral en mieles de consumo frecuente en Santa Cruz de Tenerife. Aliment equipos tecnol 1991; 9: 121-31. [ Links ]
23. De la Fuente E, Sanz ML, Martínez-Castro I, Sanz J, Ruíz-Matute AI. Volatile and carbohydrate composition of rare unifloral honeys from Spain. Food Chem 2007; 105: 84-93. [ Links ]
24. Gómez JA, García-Villanova RJ, Elvira S, Rivas T, González AM, Sánchez J. Geographical distrimination of honey through the employment of sugar pattern and common chemical quality parameters. Eur Food Res Technol 2000; 210: 437-44. [ Links ]
25. Arilla E. Estructura y metabolismo de los carbohidratos. In: Hernández M & Sastre A, editors. Tratado de Nutrición. Madrid: Díaz de Santos; 1999, pp. 53-100. [ Links ]
26. Hernández OM, Fraga JMG, Jiménez AI, Jiménez I, Arias JJ. Characterization of honey from the Canary Islands: determination of the mineral content by atomic absorption spectrophotometry. Food Chem 2005; 93: 449-58. [ Links ]
27. Yilmaz H, Yavuz O. Content of some trace metals in honey from south-eastern Anatolia. Food Chem 1999; 65: 475-6. [ Links ]
![]() Correspondence:
Correspondence:
Gara Luis.
Department of Toxicology.
University of la Laguna.
38071 Tenerife.
Canary Island. Spain.
E-mail: garaluisglez@gmail.com
Recibido: 2-VIII-2011.
1.a Revisión: 7-IX-2011.
2.a Revisión: 8-X-2011.
3.a Revisión: 17-X-2011.
4.a Revisión: 4-XI-2011.
Aceptado: 4-XI-2011.