INTRODUCTION
Obesity is associated with risk factors for chronic kidney disease, such as diabetes and hypertension, and is directly related to the development of CKD and end-stage renal disease (ESRD) 1. Worsening the situation, obesity has become a global epidemic because the population with overweight diagnoses has steadily increased over the past years 2. In Brazil, this nutritional disorder has increased in the same manner, as displayed by the data from a national expenditure survey called Survey on Family Budgets, which showed that more than 50% of the adult Brazilian population was overweight 3.
Several studies have shown that total obesity, measured by body mass index (BMI), has been associated with the development of CKD 4,5,6. Although BMI is frequently used for nutritional assessment in research, the validity of this anthropometric indicator is arguable because it only evaluates changes in body weight. Moreover, in the evaluation of risk factors associated with CKD, the pattern of distribution of body fat could be more important than an excess of adipose tissue. Some studies have reported that anthropometric indices of abdominal obesity (waist circumference [WC], waist-hip ratio [WHR] and waist stature ratio [WSR]) are more sensitive predictors of CKD 7,8,9,10. Although these anthropometric measurements have good correlations with abdominal adiposity, they do not differ visceral from subcutaneous fat.
Visceral adipose tissue (VAT), when compared to subcutaneous abdominal tissue, has shown the strongest associations with most metabolic risk factors 11. Thereby, this measurement has been associated with higher levels of blood pressure, insulin resistance and dyslipidemia 12. The reference methods for measuring VAT are computed tomography (CT) and magnetic resonance imaging (MRI). However, these methods require expensive equipment and specialized professionals, which make them unsuitable for use in primary health care 13,14. Facing these limitations, there have been validated predictive equations to estimate VAT from simple anthropometric measurement 13,15. However, there have been few studies that have used VAT to evaluate the correlation between visceral obesity and CKD (16,17).
Thus, to prevent the development of CKD in primary health care, it is important to investigate whether there are associations between different types of obesity (total, central or visceral) and CKD, as well as to identify nutritional indices that are easily performed in clinical practice and that present the strongest correlation with GFR. Therefore, the aim of this study was to evaluate the correlations between nutritional indices that measure total, central and visceral obesity and estimated glomerular filtration rate (eGFR), based on equations derived from the Modification of Diet in Renal Disease (MDRD) and Chronic Kidney Study Disease Epidemiology Collaboration (CKD-EPI) studies.
METHODS
A cross-sectional study involving hypertensive patients was performed from January 2010 to February 2011. The subjects were enrolled at the "HiperDia" Program of the Brazil's Health Ministry and were under regular follow-up in two primary health care units in the city of São Luis, Maranhão, Brazil.
Simple random sampling was performed from a list of hypertensive patients enrolled at the "HiperDia" Program from San Francisco and Cohab primary health care units. The sample size calculation was done considering the population of 550 hypertensive patients in 2010. The expected prevalence of GFR < 60 ml/min/1.73 m2 was 22% 18 in hypertensive patients, error margin of 3.5% and confidence level of 95%. The total number of hypertensive patients was estimated at 272. The sample size was increased in 16% predicting possible losses during data collection, which totalized 326 patients.
Inclusion criteria were: individuals of both sexes aged ≥ 40 years old, with diagnosed hypertension and enrolled at the "Hiperdia" Program, and who were regularly followed up at selected primary health care units. Pregnant women and patients with eGFR < 30 ml/min/1.73 m², severe cardiac insufficiency, malignancy or acquired immunodeficiency syndrome were not included. The sample included 288 patients. These exclusion criteria were justified to exclude advanced CKD patients (eGFR < 30 ml/min/1.73 m²) and young adult individuals (age < 40 years) whose underlying diseases were infectious illnesses.
The selection of hypertensive patients to compose the sample of this study was based on the fact that this pathology is one of the main independent risk factors for the progression of CKD in the Western world. Thus, it was possible to identify a greater number of people with eGFR alteration and to optimize the care provided to the population with a higher prevalence of CKD. In addition, during the statistical analysis of the data, care was taken to use hypertension as an adjustment factor in multiple linear regression, in order to evaluate the relationship between obesity and RFGe, regardless of the effect of hypertension in the individuals evaluated.
The patients responded a structured form, which collected sociodemographic, lifestyle and clinical data. At this time, blood pressure was also measured, nutritional assessment was performed, and fasting blood and urine samples were collected.
Education was assessed in years of schooling and was ranked as ≤ 8 years or ≥ 9 years. Skin color was self-reported. A person was considered as a smoker if he or she reported using nicotine in the interview, regardless of the number of cigarettes smoked. The level of physical activity was assessed by the International Physical Activity Questionnaire, a validated instrument used in Brazil that separates the days and minutes of physical activity according to leisure, locomotion, work and household chores. Physically active patients were those who performed at least 150 minutes of weekly physical activity 19.
Fasting blood samples were obtained from the participants after an overnight fast (12 hours). The blood parameters were analyzed at a certified laboratory, using standard methods and quality control procedures. Lipid profile levels (triglycerides [TG], total cholesterol [TC]; HDL cholesterol [HDLc], and LDL cholesterol [LDL-C]) were determined by enzymatic colorimetric methods. Fasting glucose (FG) and uric acid were also assessed by enzymatic methods and glycated hemoglobin was assessed by turbidimetric inhibition immunoassay. Serum creatinine (kinetic Jaffe assay) was obtained by the kinetic colorimetric method, using the Automation Roche Modular Cobas® 501. Microalbuminuria was determined by ELISA, using 24-hour urine.
The values adopted for the lipid profile were those recommended by the National Cholesterol Education Program (NCEP) 20, and for microalbuminuria, those recommended by the Kidney Disease/Improving Global Outcomes (KDIGO) study were the ones that had been used 21. For FG and glycated hemoglobin the criteria recommended by the American Diabetics Association were applied 22, which consider normal values to be less than 100 mg/dl and 7%, respectively. For uric acid, the reference value used was the recommended by the method itself, which considered the appropriate values to be ≤ 7.0 mg/dl for men and ≥ 5.7 mg/dl for women.
Renal function was assessed by eGFR equations, both based on serum creatinine, as described below. According to the KDIGO recommendations, the simplified Modification of Diet in Renal Disease (MDRD) 23 and CKD-EPI 24 equations were used as references for screening CKD. The "African-American" and "black" variables were not included in the estimation.
- Simplified MDRD equation standardized with an isotope dilution mass spectrometry 23:
Blood pressure was measured by the indirect method, using a digital sphygmomanometer (Omron HEM-705®, Japan). The patient was kept in a seated position for at least five minutes, with the arm at heart level, palm facing upward, and the elbow slightly flexed. Three measurements were obtained, with ten minute intervals between them, and the mean was calculated 25.
Anthropometric parameters included body weight, height, WC and hip circumference (HC). Body weight (in kilograms) was measured using a digital scale (Leader®, Brazil), and height (in centimeters) was measured using a portable stadiometer (Alturexata®, Brazil). Waist and hip circumferences (in centimeters) were measured using anthropometric tape (Sunny®, Brazil). Waist circumference was measured at the midpoint between the last rib and the iliac crest, during expiration. HC was gauged at the largest circumference in buttocks region, through pubic symphysis with the subject parallel to the floor.
Abdominal obesity was assessed by WC, WHR and WSR. The cut-off values for very high risk for WC were ≥ 88 cm for women and ≥ 102 cm for men. The WHR was calculated by dividing WC (cm) by HC (cm), and the cut-offs adopted were ≥ 0.85 for women and ≥ 1.00 for men. The WSR was determined by dividing WC (cm) by height (cm) and was classified as high when ≥ 0.52 in men and ≥ 0.53 in women 26.
In determining nutritional status, considering the relationship between weight and height, BMI was used, which is calculated as the ratio of weight (kg) to height (m²). The cut-off values were the recommended by the World Health Organization (WHO) 27 as follows: eutrophy (BMI between 18.5 kg/m² and 24.9 kg/m²), overweight (BMI ≥ 25.0 kg/m² and < 30.0 kg/m²), and obesity (BMI ≥ 30.0 kg/m²).
Abdominal obesity was assessed by estimated VAT from predictive equations: -453.7 + (6:37 x WC) for men and -370.5 + (4.4 x WC) + (2.62 x age) for women, as proposed by Bonora et al. 14. The equations used in this study were developed in a population with similar characteristics to those of the population included in this study. Estimated VAT was considered to be very high if ≥ 150 cm² 15.
Body fat percentage (BF %) was obtained by bioelectrical impedance analysis, which was conducted using a tetrapolar device (Biodynamics® 450, USA). The patient lay on a non-conductive surface, with legs apart and arms away from the trunk in parallel, and electrodes were placed in specific locations on the hand and on the foot of the dominant side. After entering data into the device, the test was performed, and the body composition values were immediately printed. BF% was classified according to the criteria proposed by Gallagher et al. 28 for individuals aged 40-59 years old, for whom values ≥ 28% in men and ≥ 40.0% in women were indicators of risks associated with obesity.
Aiming not to bias the test results, the study participants were instructed to follow certain prior procedures: absolute fasting for four hours, no strenuous exercise for 12 hours, and no alcohol for 48 hours. At the time of the test, they were asked to take off any metal pieces they might be wearing.
When renal function deficits (GFR < 60 ml/min/1.73 m²) were identified, patients were referred to the Clinic of Nephrology at the University Hospital of the Federal University of Maranhão, which is reference in the city of São Luís due to its specialized evaluation and treatment.
Qualitative variables are presented as frequencies and percentages and quantitative variables, as the means and standard deviations (means ± SD). Normality of the quantitative variables was analyzed by the Shapiro-Wilk test. For comparison of demographic, clinical, laboratory and nutritional indices by sex, Student's t test or the Mann-Whitney test were used. Comparisons between nutritional indices, according to BMI categories, were performed using the Kruskal-Wallis test or analysis of variance (ANOVA).
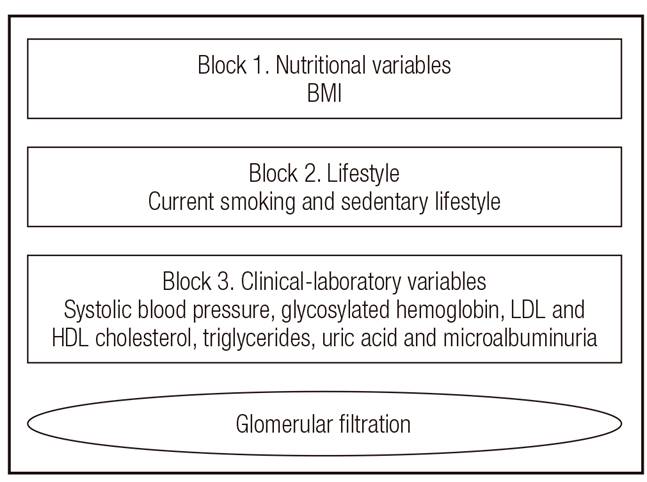
For associations between nutritional indices (BMI, WC, WHR, WSR, eVAT and BF %) and eGFR by the MDRD and the CKD-EPI equations, simple linear regression analysis was conducted. Then, to identify potential confounding factors (BMI, smoking and physical inactivity) and mediators (systolic blood pressure - SBP, Hb1c, LDLc, HDLc, TG, uric acid and microalbuminuria) in the relationships between nutritional indices and eGFR, a multiple linear regression analysis model was used with a hierarchical approach (Fig. 1). In each block of the analysis, only variables that showed p-values < 0.10 remained. In the final model, only variables with p-values < 0.05 were considered. Data were analyzed using the STATA program (version 10.0).
The study was approved by the Research Ethics Committee of the Federal University of Maranhão, Brazil (Protocol 312/2009). Patients who agreed to participate provided written informed consent.
RESULTS
There were 288 hypertensive patients evaluated. The mean age was 61.3 (± 10.1) years old. Women predominated (76.4%), and most of the study participants reported less than eight years of education (73.6%) and that their skin color was not white (82.3%).
The mean duration of hypertension was 10.4 (± 9.2) years. The mean level of SBP was 144.7 (± 22.4) mmHg and the mean level of diastolic blood pressure (DBP) was 82.0 (± 13.2) mmHg. The prevalence of reduced eGFR (< 60 ml/min/1.73 m²) was 16.7% and 19.8%, by the MDRD and the CKD-EPI equations, respectively (Table 1).
Table I. Clinical, biological and nutritional characteristics of study participants, according to sex

eTAV: estimated visceral adipose tissue; eGFR MDRD: estimated glomerular filtration rate by Modification of Diet in Renal Disease equation (ml/min/1.73 m²); eGFR CKD-EPI: estimated glomerular filtration rate by Chronic Kidney Disease Epidemiology Collaboration equation (ml/min/1.73 m²). Data are expressed as the means ± SDs.
The prevalence of diabetes in the study participants was 33.1%, and there were no statistically significant differences in age, sex, BMI, WC or eGFR by the MDRD and the CKD-EPI equations between hypertensive patients with and without diabetes (data not shown in table).
The mean and standard deviation of BMI (27.8 ± 4.7 kg/m²) were in the overweight range. Obese patients accounted for 29.5% and overweight patients for 41.7%. Men and women showed mean WHRs in the high risk range and visceral obesity by eVAT in the very high risk range. Only women presented WC in the very high risk range (Table 1).
When the nutritional indices were compared by sex, women had higher BF% (35.8 ± 3.7% vs 25.4 ± 4.4%, p < 0.001). However, men presented higher mean WC (98.2 ± 9.1 cm vs 93.8 ± 11.2 cm, p = 0.003) and mean WHR (1.01 ± 0.05 vs 0.93 ± 0.07, p < 0.001); when considering the values recommended by the WHO 27, women showed more altered levels (Table 1).
In the study sample, the mean serum levels of FG, TC and TG were classified by risk range. Only women had mean LDL-c in the risky range, and only men presented mean microalbuminuria in the range of risk. Compared by sex, men had lower serum levels of HDL-c (41.4 ± 12.7 mg/dl vs 49.8 ± 13.0 mg/dl, p < 0.001) and higher microalbuminuria (39.1 ± 92.8 mg/24 h vs 16.0 ± 36.4 mg/24 h, p = 0.011), uric acid (6.1 ± 1.7 mg/dl vs 5.0 ± 1.4 mg/dl, p < 0.001) and serum creatinine (1.06 ± 0.28 mg/dl vs 0.79 ± 0.17 mg/dl, p < 0.001), while women exhibited higher serum TC (226 ± 47.9 mg/dl vs 204.7 ± 44.3 mg/dl, p < 0.001) and serum levels of LDL-c (143.6 ± 41.5 mg/dl vs 127.5 ± 38.1 mg/dl, p = 0.004) (Table 1).
When BMI classification (eutrophic, overweight and obesity) was analyzed, the mean nutritional indices differed significantly, except for eVAT and BF% in men and WHR in women. There was no statistically significant difference between mean eGFR by MDRD and mean eGFR by CKD-EPI (Table 2).
Table II. Nutritional indices and eGFR evaluation, according to body mass index category

BMI: body mass index ranged as eutrophic (18.0-24.9 kg/m²), overweight (25.0-29.9 kg/m²) and obesity (≥ 30 kg/m²); WC: waist circumference; WHR: waist-hip ratio; WSR: waist-to-stature ratio; WHR: waist-hip ratio; eVAT: estimated visceral adipose tissue; eGFR MDRD: estimated glomerular filtration rate by Modification of Diet in Renal Disease equation (ml/min/1.73 m²); eGFR CKD-EPI: estimated glomerular filtration rate by Chronic Kidney Disease Epidemiology Collaboration equation (ml/min/1.73 m²); data are expressed as the means ± SD.
*p value not applicable;
†p value < 0.05 vs eutrophic vs overweight;
‡p value < 0.05 vs eutrophic vs obese,
§p value < 0.05 vs overweight.
Table 3 shows the univariate analysis for associations between nutritional indices and eGFR. In women, WHR and eVAT were negatively correlated with eGFR by MDRD (Coef = -35.60, p = 0.040; Coef = -0.08, p < 0.001, respectively) and CKD-EPI (Coef = -56.89, p = 0.027; Coef = -0.14, p < 0.001, respectively). Other nutritional indices (BMI, WC, WSR and BF %) did not show any associations. Among men, no correlations were found between nutritional indices and eGFR by the MDRD or CKD-EPI equations.
Table III. Nutritional indices and eGFR simple linear regression analysis, according to sex

eGFR MDRD: estimated glomerular filtration rate by Modification of Diet in Renal Disease equation (ml/min/1.73 m²); eGFR CKD-EPI: estimated glomerular filtration rate by Chronic Kidney Disease Epidemiology Collaboration equation (ml/min/1.73 m²); BMI: body mass index; WC: waist circumference; WHR: waist-hip ratio; WSR: waist-to-height ratio; eVAT: estimated visceral adipose tissue; BF: body fat; Coef: coefficient of the linear regression model.
Table 4 displays the nutritional indices that were significantly associated with eGFR, after adjustment for BMI, physical inactivity, current smoking, SBP, glycated hemoglobin, LDLc, HDLc, uric acid and microalbuminuria. In women, eVAT remained associated with eGFR by the MDRD (Coef = -0.06, p-value = 0.008) and CKD-EPI equations (Coef = -0.11, p-value = 0.002). After adjustment for clinical and laboratory variables, WHR was not associated, and the other nutritional indices continued not to show significant associations. In men, the lack of associations between nutritional indices and the two equations for eGFR persisted.
Table IV. Multiple linear regression analysis for association between nutritional indices and eGFR

eGFR MDRD: estimated glomerular filtration rate by Modification of Diet in Renal Disease equation (ml/min/1.73 m²); eGFR CKD-EPI: estimated glomerular filtration rate by Chronic Kidney Disease Epidemiology Collaboration equation (ml/min/1.73 m²); WHR: waist-hip ratio; eVAT: estimated visceral adipose tissue; Coef: coefficient of the linear regression model. Block 1: adjusted by overweight: body mass index. Block 2: djusted by lifestyle: sedentary and current smoking. Block 3: adjusted by clinical and laboratory variables: systolic blood pressure, glycated hemoglobin, LDL and HDL cholesterol, triglycerides, uric acid and microalbuminuria.
Figure 2 demonstrates the associations between eVAT and eGFR by the MDRD and CKD-EPI equations in hypertensive women by BMI category. Estimated VAT was significantly associated with eGFR by MDRD and CKD-EPI in overweight and obese patients. In eutrophic women, eVAT was associated with eGFR only by the CKD-EPI equation.
DISCUSSION
This paper evaluated the associations between nutritional indices to measure overall, central and visceral obesity and eGFR by the MDRD and CKD-EPI equations in hypertensive patients. In men, there were no correlations between eGFR and nutritional indices; however, in women, eVAT and WHR were negatively correlated with eGFR by the MDRD and the CKD-EPI equations.
When the factors that could affect the association between nutritional indices and eGFR were considered together, after adjustment, only eVAT remained negatively correlated with eGFR by the MDRD and CKD-EPI equations, independent of BMI, smoking, sedentary behavior, SBP, glycated hemoglobin, LDL and HDL cholesterol, uric acid and microalbuminuria. WHR had already lost its association with eGFR after adjustment for clinical and laboratory variables. It is worth mentioning that multiple regression analysis was performed only for females because it was the one that showed an association between eGFR and nutritional indexes.
The role of body fat in the development of CKD remains unclear. Several studies have investigated this association based on BMI, but the results have been conflicting. Some studies have found a positive association between high baseline BMI and renal dysfunction 4,6, while others have observed relationships between the increasing being overweight at 20 years of age and an increased risk of CKD, although no association was found with excess weight at the time of the interview 29.
However, other studies have shown an inverse 30, J-shaped 31 or no association 32,33 between BMI and kidney function. Ramírez et al. 31, in a study with 189,117 Asian adults, observed a J-shaped relationship between proteinuria and extremes of BMI, i.e., subjects with BMI ≤ 18.0 or ≥ 25.0 kg/m² had higher proteinuria compared with those with BMI > 18.0 and < 25.0 kg/m². Gerchman et al. 30, in a cross-sectional study with 268 Japanese-Americans (mean age > 60 years) who had BMI and WC in normal ranges, noted that creatinine clearance decreased concomitantly with BMI, and they attributed this relationship to lean body mass.
The disagreement among different studies that have attempted to explain the relationship between BMI and CKD can be attributed to study designs that did not consider the effects of reverse causality. It could also be ascribed to the limitations of BMI as an indicator of body composition. Despite being the anthropometric indicator most often used in research to classify obesity, this parameter is not able to distinguish lean from fat mass, to differentiate the distribution of body fat, and to detect the increase in fat that occurs with advancing age 27.
In this study, BMI was not associated with eGFR. This finding could be assigned to the limitation of this index as an indicator of obesity because nutritional indices for abdominal obesity and visceral fat have been classified in the range of high or very high risk, especially in women, whereas the patients had a mean BMI representative of overweight (27.8 ± 4.7 kg/m²).
In addition, measurements of WC, WHR and WSR have been widely used in epidemiological studies to evaluate abdominal obesity. However, few of these studies have been conducted in CKD. Researchers have shown that WC 8,34, WHR 7,10 and WSR 9 are more sensitive predictors of CKD and might be more important than excess adipose tissue in the evaluation of metabolic effects and risk factors associated with obesity and disease processes 35,36,37.
A prospective study conducted with over 24,500 participants in the United Kingdom showed that WHR was independently and more consistently correlated with the prediction of coronary heart disease than BMI or WC 36. Similar evidence was observed in another study performed in the Netherlands with 7,676 individuals 10 and another in Poland with 730 female subjects, aged 65-80 7, correlating WHR with kidney function.
The present study noted an association between WHR and eGFR, regardless of overweight or obesity, smoking and sedentary habits, but this association did not subsist after adjustment for clinical and laboratory variables. This finding could suggest that WHR has an indirect relationship with eGFR, mediated by metabolic changes that are risk factors for CKD.
Although WC, WHR and WSR provide better evaluations of central adiposity than BMI, the lack of association found in this study was possibly due to the inability of those measurements to distinguish the body's adipose tissue compartment, i.e., abdominal subcutaneous fat, from abdominal visceral fat. Another justification that also might have contributed to this lack of association between WSR and eGFR was the rating of 92.4% of hypertensive patients in the high risk range, which reduced the power of the study to identify this association.
Noori et al. 8 reported that physiological changes in obesity, to some extent, depended on the regional distribution of adipose tissue. Although abdominal fat is the sum of VAT and subcutaneous adipose tissue, in a study with 3,001 participants from the Framingham Heart Study, Fox et al. 11 reported that VAT, measured by CT, was more strongly associated with adverse effects on metabolic risk profile, even considering anthropometric standards. In addition, Pou et al. 38 undertook a study with 1,250 individuals and concluded that the effects of visceral fat could not be completely explained by clinical measurements of obesity, such as BMI and WC.
In this study, the eVAT was negatively associated with eGFR in women, independent of BMI, smoking, sedentary behavior, SBP, glycated hemoglobin, HDLc, LDLc, uric acid and microalbuminuria. This finding suggests that accumulation of adipose tissue in visceral regions could contribute to renal damage in women. Nevertheless, this association was not observed in men. A possible explanation for this result is the sample size of men, which was only sufficient to detect significantly high magnitude correlations (close to 1).
Acknowledging these findings, two studies in Korea which measured VAT using multi-frequency bio-impedance 16 and CT 17 also found an association with GFR, supporting the role of visceral fat in GFR reduction. Kang et al. 16 evaluated 22,480 adults at a university hospital and verified sensitivity and specificity of VAT to predict CKD. Kim et al. 17 measured the VAT of 929 type 2 diabetes patients and found an association with eGFR estimated by the MDRD equation. Furthermore, Fox et al. 11 also noted that the relationships between VAT and metabolic risk factors were consistently stronger in women than in men.
It is important to emphasize that multi-frequency bio-impedance and CT are costly techniques, which prevent their application in studies involving a large number of individuals, especially those conducted "in the field". Overcoming these limitations, some studies have developed and evaluated the equations for predicting VAT from simple anthropometric measures using multiple regression analysis 13,14. It is worth highlighting that despite the equations that had been used had not been validated for the studied population, they were used in studies with individuals similar to those investigated in this study.
The association between obesity and kidney disease is complex and yet not fully understood. Excess fat accumulation is responsible for high levels of inflammation and oxidative stress, which have deleterious renal effects 1. In addition, abdominal visceral obesity was associated with increase in blood pressure, insulin levels, insulin resistance and dyslipidemia 12.
Visceral obesity, when associated with insulin resistance, leads to compensatory hyperinsulinemia and inappropriate activation of the renin-angiotensin system and to kidney oxidative stress, which results in increased blood pressure 39, excess of aldosterone, glomerular hypertension, endothelial dysfunction and vasoconstriction 8. In addition to these effects, visceral adiposity can physically compress the kidneys, increasing intra-renal pressure and tubular reabsorption. The effects of renal injury are continuing GFR loss, increased blood pressure and worse cardiovascular morbidity and mortality 5.
As a limitation of this study, because it is a cross-sectional one, it was not possible to establish the effects of obesity over time. However, an association was identified between eGFR and eVAT in hypertensive women. While in this study a gold standard method to evaluate GFR was not used, it was estimated by the CKD-EPI and the MDRD equations, which have better accuracy and less bias, particularly in the range of GFR ≥ 60 ml/min/1.73 m², which is consistent with the study. Another limitation was the absence of the analysis of the inflammatory condition, but the higher production of proinflammatory cytokines is inherent to excess body fat.
As an advantage, VAT was used as a measurer of the body fat, which is strongly associated with most of the metabolic risk factors. Despite VAT being estimated by equation, these were developed in a population with similar characteristics to this study and the results of this study agree with those that evaluated the associations of VAT, measured by multi-frequency bio-impedance 16 and CT 17, with GFR and with disease processes 38,40.
CONCLUSION
Estimated VAT was negatively associated with eGFR by the MDRD and the CKD-EPI equations only in women, and WHR did not remain associated after adjustment for clinical and laboratory variables. Other nutritional indices were not correlated with eGFR. In clinical practice, estimating VAT could improve identification in primary health care of hypertensive women decreased risk factors for developing CKD.












 Curriculum ScienTI
Curriculum ScienTI