Meu SciELO
Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO  Similares em Google
Similares em Google
Compartilhar
Revista Española de Enfermedades Digestivas
versão impressa ISSN 1130-0108
Rev. esp. enferm. dig. vol.96 no.2 Madrid Fev. 2004
| ORIGINAL PAPERS |
Prognostic significance of the p185 protein in colorectal cancer
M. T. Sanz-Casla, M. Vidaurreta, M. L. Maestro, J. A. López1, F. Jiménez2, C. Fernández3,
C. Maestro4, J. L. Balibrea2 and F. J. Cerdán2
Service of Clinical Biochemistry. Tumor Biology Section. 1Department of Pathology. 2Service of General Surgery II.
3Research Unit. Hospital Clínico San Carlos. 4Department of Morphological Sciences I. Faculty of Medicine.
Universidad Complutense. Madrid, Spain
ABSTRACT
The amplification and/or overexpression of the c-erbB-2/neu oncogene may play a role in tumor development and progression. The aim of this prospective study was to evaluate the prognostic value of p185 protein in colorectal cancer using immunohistochemical techniques. We analyzed 106 colorectal tumor tissue specimens from patients who had been operated on by the same surgeon and subjected to a median follow-up of 3 years. Thirty-three per cent of patients showed p185 overexpression related to an advanced stage of the disease. In patients with adenocarcinoma tumors of the colon without distant metastases, p185 detection was found to be of clinical prognostic relevance (p = 0.06).
Key words: c-erbB-2/neu oncogene. p185 protein. Colorectal carcinoma. Prognosis.
Sanz-Casla MT, Vidaurreta M, Maestro ML, López JA, Jiménez F, Fernández C, Maestro C, Balibrea JL, Cerdán FJ. Prognostic significance of the p185 protein in colorectal cancer. Rev Esp Enferm Dig 2004; 96: 102-109.
This study was funded by Project Nº 08.1/0012/1999 of Comunidad de Madrid.
Recibido: 23-06-03.
Aceptado: 12-09-03.
Correspondencia: Mª Luisa Maestro de las Casas. Servicio de Análisis Clínicos. Hospital Clínico San Carlos. C/ Martín Lagos, s/n. 28040 Madrid. Tel.: 91 3303171. Fax: 91 3303179. e-mail: mmaestro@hcsc.es
INTRODUCTION
The c-erbB-2/neu proto-oncogene belongs to a family of receptors with tyrosine-kinase activity. Located on chromosome 17q21, this oncogene encodes for a 185 Kda transmembrane glycoprotein that is similar in structure to the epidermal growth factor receptor, which induces cell proliferation and differentiation (1,2).
Gene amplification, the most common of all genetic alterations, leads to the overexpression of proteins, although protein overexpression is not always the result of gene amplification (3). The amplification and/or overexpression of c-erbB-2/neu may give rise to uncontrolled cell proliferation causing tumor development and progression (4). This alteration may be evaluated by gene or protein analysis. In most cases, fluorescence in situ hybridization (FISH) is used for genetic studies while investigations on the protein usually involve qualitative techniques such as immunohistochemistry (IHC). In situ hybridization and immunohistochemistry show 92% agreement between results (5), enabling an accurate comparison or confirmation of results.
c-erbB-2/neu amplification and/or overexpression has been associated with a worse prognosis in tumors of the breast (6,7), ovary (6), and stomach (8,9). Most studies of the c-erbB-2/neu oncogene in colorectal tumors have involved the detection of the p185 protein using IHC techniques. These studies report protein overexpression rates of 35 to 100% (10-12), and there are many doubts concerning the prognostic significance of this oncogene in this disease (13-16).
The aim of this study was to assess the prognostic value of protein p185 using IHC techniques in patients diagnosed with colorectal carcinoma.
PATIENTS AND METHODS
Patients
This prospective cohort study was performed on 106 patients undergoing surgery for primary colorectal cancer at Surgery Unit II of the "Hospital Clínico San Carlos", Madrid, over the period 1995 to 1998. All the patients were operated on by the same surgeon who undertook a radical oncological resection procedure according to tumor site. Surgery was defined as curative when there were no macroscopical signs of residual tumor after resection. According to this criterion, a curative resection was performed in 84 patients (80%), and in 22 patients (20%) the tumor was resected as palliative treatment. Patients were excluded from our study if they had metachronic carcinoma, familial polyps or intestinal inflammatory disease. No patient received neoadjuvant treatment. An informed consent was obtained from the patients before surgery. The protocol was approved by the hospital ethics and clinical research committee. Patient follow-up was performed according to a protocol developed at our unit (17). Tumors were stratified according to Dukes' classification. Patients with stages B, C or D under 80 years of age were given 5-fluorouracil (5-FU) and leucovorin as adjuvant chemotherapy. These patients accounted for 75% of the study population.
Methods
Specimen processing
Tumor specimens were obtained during surgery and placed in formalin for subsequent embedding in paraffin. The anatomopathological examination was independently conducted by two pathologists especially for this study. It was confirmed that all tumor specimens showed at least 80% tumor cells.
Immunohistochemical determination of the p185 protein
The specimens used for IHC were 7 mm-thick tissue sections embedded in paraffin. Once deparaffinated in xylene and dehydrated in alcohol, the sections were stained using the peroxidase-antiperoxidase conjugate method. We used a rabbit monoclonal antibody against the p185 protein (DAKO K5206). After incubating for 30 minutes at room temperature with the primary antibody, the sections were incubated with a biotinylated secondary antibody and the peroxidase-antiperoxidase complex. Labelling was visualized by further incubation with 0.02% hydrogen peroxide and 0.6 mmol/L diaminobenzidine in methanol for 10 minutes at room temperature. As a positive control, we used a breast tumor tissue section showing p185 overexpression. A glass slide bearing PBS buffer was used as the negative control.
To establish the prognostic value of the p185 protein, the percentages of cells showing staining in the cytoplasm and/or cell membrane were distributed as percentiles. Then, using Cox's proportional hazards model, we established the value that best discriminated the risk of death for patients with colorectal cancer. A determination was taken as being positive when over 20% of the cells showed cytoplasmic and/or membrane staining.
Statistical analysis
Qualitative variables are provided with their frequency distributions. Quantitative variables are expressed as mean, standard deviation (SD) and range. The Chi-squared test was used to evaluate associations between qualitative variables. When more than 25% of the expected associations were under 5, Fisher's exact test was used. Overall survival (OS) and disease-free survival (DFS) rates were determined by the Kaplan-Meier method. Breslow's exact test was used to compare survival functions among groups. The event in OS was defined as deaths produced as a consequence of the tumor, excluding patients alive and those dying from another cause. Overall survival was calculated as the time between the day of surgery and that of death or last follow-up. The event in DFS was defined as the diagnosis of local or distant recession in patients previously free of disease, that is, all those undergoing curative surgery. Disease-free survival was calculated as the time from surgery to diagnosis of the first recession. A Cox's proportional hazards regression model was fitted to relate the prognostic factors in the univariate and multivariate analyses. In each contrast, the null hypothesis was rejected for a type I error below 0.05. All statistical tests were performed using the SSPS version 11.5 software.
RESULTS
Of the 106 patients included in our study, 50.9% were males and 49.1% were females. Mean patient age was 69 years (SD ±12), ranging from 35 to 91 years. Table I shows the clinical-pathological features of the population. In all, 52.8% of tumors were located in the colon and 47.2% in the rectum; 5.7% were mucinous adenocarcinomas.
Thirty-three per cent of patients (35/106) showed overexpression of the p185 protein. This protein was found to be related to tumor presence, with percentage p185 positivity being lower at early stages than at more advanced stages (p < 0.001) (Table I).
Postoperative course. Overall survival
The median length of follow-up was 37 months (3 years), with an interquartile range of 8 to 69 months.
The overall survival rate of our patient series was 67% at 3 years. All survival analyses are referred to our median follow-up time. During follow-up, 43 patients died as a consequence of the neoplasm. One patient was lost to follow-up.
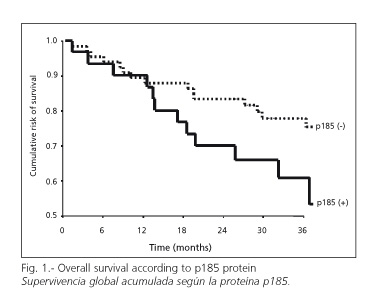
OS were 72% for patients showing negative p185, and 51% for those showing a positive result (p = 0.08) (Fig. 1). When rates were stratified according to tumor site, OS was greater (82%) for patients with rectal tumors and positive p185 than for those with negative p185 (62%) (p = 0.05). According to histological type, patients with adenocarcinomas showed an OS rate of 76% if their p185 was negative, and one of 53% when they showed positivity for this protein (p = 0.09).
Variables included in the multivariate analysis were: p185 protein, gender, degree of differentiation, stage A, B or C, and histological type of adenocarcinoma. Patients with stage D tumors were excluded since prognostic information is of scarce value. Those with mucoid adenocarcinomas were also excluded since they accounted for 5% of the tumors and disease progression is more aggressive. This analysis was independently performed on patients with tumors of the colon or rectum, since OS significantly differs between these groups. In patients with tumors of the rectum, the p185 protein was of no prognostic value independently of the remaining variables. In those with tumors of the colon, p185 proved to be of clinical significance: the relative risk of death shown by patients overexpressing the protein was 3.9 times greater than those with a negative p185 result (p = 0.06) (95% CI = 0.9-16.4).
Postoperative course. Disease-free survival
DFS rate in our patient series was 75%. During follow-up, tumor recurrence occurred in 19 patients, including 8 patients with stage D tumors in whom it was possible to perform curative surgery. Recurrences were local in 5 patients (26.3%) and distant in 14 patients (73.7%).
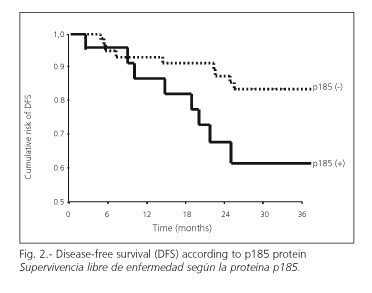
DFS rates of patients showing negative p185 (82%) were higher than those with positive p185 (58%) (p = 0.02); the relative risk of recurrence was 3.3 times greater in patients overexpressing the protein (p=0.03) (95%CI=1.2-10.0) (Fig. 2). When results were stratified according to tumor site, patients with tumors of the colon with negative p185 showed higher DFS (83%) than those with a positive p185 result (54%) (p = 0.08). When stratified by histological type, the OS of patients with adenocarcinomas and negative p185 was 82%, higher than for those expressing p185 (56%) (p = 0.01).
In the multivariate analysis of patients with adenocarcinomas of the colon staged as A, B or C, the p185 result was clinically relevant yet not significant: the relative risk of recurrence in patients overexpressing p185 was 3.3 times higher than in those with a negative p185 result (p = 0.09) (95% CI = 0.8-13.6).
DISCUSSION
Most studies on the c-erbB-2/neu oncogene in colorectal cancer have determined protein overexpression using IHC techniques. However, different authors do not seem to have a standard criterion for defining overexpression of the oncogene. Thus, published rates of p185 overexpression in patients with colorectal tumors range from 35 to 100% (10-12).
Most authors would agree that there is no significant relationship between p185 overexpression and the age or gender of the patient, nor the site or histological type of the tumor (10-12). Several authors have reported that overexpression of c-erbB-2/neu can predict the course of several human cancers such as stomach (8,9) and breast (6,7). However, there are many doubts about the prognostic significance of this oncogene in colorectal tumors (13-16). While authors such as McKay et al. (18) or Webb et al. (19) found that c-erbB-2/neu was of no prognostic value, others including Kay et al. (10), Kapitanovic et al. (11), and Osako et al. (12) described this oncogene as an independent prognostic factor in this disease. These authors argue that overall survival is significantly lower in patients who show c-erbB-2/neu overexpression compared to those who do not express this protein. Further, it seems that p185 overexpression is related to Dukes' classification, in such a way that patients with tumors staged as A or B show significantly lower overexpression (p < 0.05) than those with stage C or D tumors (11).
In the present study, we adopted the criterion of 20% of cells showing cytoplasmic and/or membrane staining as indicative of p185 protein overexpression. This assumption is consistent with most investigations performed in this and other tumor types (7,10,15). Although p185 is a transmembrane receptor, we were able to detect this protein in the cytoplasm as well as on the cell membrane, since it is at the level of the cytoplasm that the post-transcriptional alteration occurs (20).
In all, 33% of our patient population overexpressed p185 and the incidence of overexpression was significantly higher for stage C and D tumors when compared to the earlier stages A and B, likely pointing to a significant role for the c-erbB-2/neu oncogene in the progression of colorectal tumor genesis. In colon adenocarcinomas with no evidence of distant metastasis at the time of diagnosis, p185 overexpression increases the risk of death by four times, though this association was clinically relevant yet not significant.
In the search for new biological prognostic factors, it should be borne in mind that the prognosis of colorectal cancer not only depends on clinical factors such as tumor stage, but is also affected by factors as specific as the surgeon's experience (21). This undermines the results emerging from many series that might not represent ideal management of the disease. We tried to eliminate this type of bias by only including patients operated on by the same surgeon. Nevertheless, our study design and population size precludes any conclusions of further clinical significance.
Despite the fact that the p185 protein was related to clinical prognosis, this link was not statistically significant. To date, evaluating the overexpression of the c-erbB-2/neu oncogen in colorectal cancer has only served to improve our understanding of tumor biology and to open a new path in the search for treatment options in experimental models.
REFERENCES
1. Yamamoto T, Ikawa S, Akiyama T, et al. Similarity of protein encoded by the human c-erbB-2 gene to epidermal growth factor receptor. Nature 1986; 319: 230-4. [ Links ]
2. Hung MC, Lau YK. Basic science of HER-2/neu: a review. Semin Oncol 1999; 26: 51-9. [ Links ]
3. Lemoine LR, Jain S, Silvestre F, Lopes C, Hughes CM, McLelland E, et al. Amplifiaction and overexpression of the EGF receptor and c-erbB-2 proto-oncogenes in human stomach cancer. Br J Cancer 1991; 64: 79-83. [ Links ]
4. Klapper LN, Kirschbaum MH, Sela M, Yarden Y. Biochemical and clinical implications of the ErbB/HER signalling network of growth factor receptors. Adv Cancer Res 2000; 77: 25-79. [ Links ]
5. Bozzeti C, Nizzoli R, Guazzi A, Flora M, Bassano C, Crafa P, et al. HER-2/neu amplification detected by fluorescence in situ hybridization in fine needle aspirates from primary breast. Ann Oncol, 2002; 13: 1398-403. [ Links ]
6. Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 1989; 244: 707-12. [ Links ]
7. Gusteson BA, Price KN, Gelber RD, Goldhirsch A, Säve-Söderborgh AR, Styles J, et al. Prognostic importance of c-erbB-2 expression in breast cancer. J Clin Oncol 1992; 10: 1049-56. [ Links ]
8. Amadori D, Maltoni M, Volpi A, Nanni O, Scarpi E, Renault B, et al. Gene amplification and proliferative kinetics in relation to prognosis of patients with gastric carcinoma. Cancer 1997; 79: 226-32. [ Links ]
9. Yonemora Y, Ninomiya I, Tsugawa K, Fushida S, Fujimura T, Miyazaki I, et al. Prognostic significance of c-erbB-2 gene expression in the poorly differentiated type of adenocarcinoma of the stomach. Cancer Detect Prev 1998; 22: 139-46. [ Links ]
10. Kay EW, Mulcahy H, Walsh CB, Leader M, O'Donoghue D. Cytoplasmic c-erbB-2 protein expression correlates with survival in Dukes'B colorectal carcinoma. Histopathology 1994; 25: 455-61. [ Links ]
11. Kapitanovic S, Radosevic S, Kapitanovic M, Andelinovic S, Ferencic Z, Tavassolo M, et al. The expression of p185 HER-2/neu correlates with the stage of disease and survival in colorectal cancer. Gastroenterology 1997; 112: 1103-13. [ Links ]
12. Osako T, Miyahara M, Uchino S, Inomata M, Kitano S, Kobayashi M. Inmunohistochemical Study of c-erbB-2 protein in colorectal cancer and the correlation with patient survival. Oncology 1998; 55: 548-55. [ Links ]
13. Yokota J, Yamamoto T, Toyoshima K, Terada M, Sugimura T, Battifora H, et al. Amplification of c-erbB-2 oncogene in human adenocarcinomas in vivo. Lancet 1986; i: 765-6. [ Links ]
14. McCann A, Dervan PA, Johnston PA, Gullick WJ, Carney DN. c-erbB-2 oncoprotein expression in primary human tumors. Cancer 1990; 65: 88-92. [ Links ]
15. D'Emilia J, Bulovas K, D'Ercole B, Wolf B, Steele G Jr, Summerhayes IC. Expression of the c-erbB-2 gene product (p185) at different stages of neoplastic progression in the colon. Oncogene 1989; 4: 1233-9. [ Links ]
16. Ohmori K, Uchino J, Kondou Y, et al. Prediction of hepatic metastasis after resection of colorectal carcinoma-From the viewpoint of pathologic features. C-erbB-2 expression and nuclear DNA content. Jpn J Gastroenterol Surg 1991; 24: 2963-9. [ Links ]
17. Cerdán FJ. Seguimiento de los pacientes intervenidos por cáncer colorrectal. Rev Cancer 1997; 11: 32-41. [ Links ]
18. McKay JA, Loane JF, Ross VG, Ameyaw MM, Murray GI, Cassidy J. c-erbB-2 is not a major factor in the development of colorectal cancer. Br J Cancer 2002; 86: 568-73. [ Links ]
19. Webb A, Scott-Mackie P, Cunningham D, Norman A, Andreyev J, O'Brien M. The prognostic value of CEA, beta HCG, AFP, CA125, CA19-9 and C-erb B-2, beta HCG immunohistochemistry in advanced colorectal cancer. Ann Oncol 1995; 6: 581-7. [ Links ]
20. Corbett IP, Henry JA, Angus B, Watchorn CJ, Wilkinson L, Hennessi C, et al. NCLA-CB11, a new monoclonal antibody recognizing the internal domain of the c-erbB-2 oncoge protein effective for use on formalin-fixed, paraffin-embedded tissue. J Pathol 1990; 161: 15-25. [ Links ]
21. Cerdán J. Recidiva locorregional en el cáncer de recto. Cir Esp 2003; 73: 63-7. [ Links ]











 texto em
texto em