Meu SciELO
Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Revista Española de Enfermedades Digestivas
versão impressa ISSN 1130-0108
Rev. esp. enferm. dig. vol.97 no.11 Madrid Nov. 2005
| ORIGINAL PAPERS |
Role of intestinal bacterial overgrowth and intestinal motility in bacterial translocation
in experimental cirrhosis
E. Sánchez, F. Casafont, A. Guerra, I. de Benito1 and F. Pons-Romero
Services of Gastroenterology and 1Microbiology. Hospital Universitario Marqués de Valdecilla. Santander, Spain
ABSTRACT
Background: intestinal bacterial overgrowth (IBO) is related to small bowel motility and has been involved in the pathogenesis of bacterial translocation (BT) in experimental models, and both overgrowing gut flora and translocating bacteria to mesenteric lymph nodes are common features in cirrhosis.
Objectives: the aims of this study were to analyze cecal aerobic bacteria and intestinal transit in cirrhotic rats, and their relationship with BT, evaluating the role of intestinal bacterial overgrowth and small bowel dismotility in the development of BT in experimental cirrhosis.
Material and methods: we included twenty-seven male Sprague-Dawley rats with carbon tetrachloride-induced cirrhosis without ascites and ten controls. Cultures of mesenteric lymph nodes (MLN), peripheral and portal blood, liver, spleen and cecal samples were carried out. Small intestinal transit was determined in ten cirrhotic rats and in ten control rats.
Results: the prevalence of bacterial translocation was 56%. Total cecal aerobic bacteria count was significantly higher in cirrhotic rats than in control rats (p < 0.001). Cirrhotic rats with translocated bacteria had higher total aerobic intestinal counts than culture-negative MLN bacteria (p < 0.05). The prevalence of total intestinal bacterial overgrowth in cirrhotic animals was 67%, and 0% in control animals (p < 0.001). According to BT, total IBO was more frequent in cirrhotic rats with BT versus those without BT (93 vs. 33%) (p < 0.001). Of the translocating bacteria, 95.6% were found to be overgrown in the cecum. The small-intestinal transit was slower in cirrhotic rats (60.5 ± 12.7 cm vs. 81.2 ± 5.7 cm) than in control animals (p < 0.001).
Conclusions: these results suggest that the increase of intestinal aerobic bacteria in experimental cirrhosis is associated with translocation. In addition, IBO is frequent in cirrhotic rats, and is supposed to play an important role in the development of BT. Impaired motility of the small intestine is a common feature in cirrhosis and may be implicated in the pathogenesis of IBO.
Key words: Intestinal bacterial overgrowth. Bacterial translocation. Experimental cirrhosis. Intestinal motility.
Sánchez E, Casafont F, Guerra A de Benito I, Pons- Romero F. Role of intestinal bacterial overgrowth and intestinal motility in bacterial translocation in experimental cirrhosis. Rev Esp Enferm Dig 2005; 97: 805-814.
This work has been supported by a grant (C03/02) from FIS-ISCIII, "Red Nacional de Investigación en Hepatología y Gastroenterología" (RHIGH).
Recibido: 05-05-05.
Aceptado: 31-05-05.
Correspondencia: Fernando Casafont Morencos. Servicio de Aparato Digestivo, Hospital Universitario Marqués de Valdecilla. Avda. de Valdecilla, s/n. 39008 Santander. e-mail: digcmf@humv.es
INTRODUCTION
The passage of viable flora from the gastrointestinal tract to the mesenteric lymph nodes (MLN) is known as bacterial translocation (BT) (1). This process has been documented to occur in numerous clinical situations, and has been associated with the development of sepsis by gram-negative bacteria (2).
Infections are frequent in the setting of cirrhosis, and organisms of enteric origin cause most of them (3). Several studies have documented the importance of BT in cirrhosis (4,5), and it appears to play a key role in the pathogenesis of spontaneous bacterial peritonitis (SBP) (6,7). The mechanisms promoting the translocation of the gut flora in cirrhosis are not totally understood, although endotoxemia and malnutrition have both been involved (8-10). The disruption of the gut flora equilibrium is related to the development of BT (11,12). Intestinal bacterial overgrowth (IBO) has been reported in cirrhotic patients and related to the development of SBP (13-16). In addition, selective intestinal decontamination with suppression of aerobic intestinal bacteria prevents BT in cirrhotic rats, and is the current prophylaxis for SBP in humans (17-20). On the other hand, several studies have shown that impaired intestinal motility is one of the factors contributing to enteric bacterial overgrowth, and also that intestinal transit is delayed in the setting of cirrhosis mainly related to the severity of disease (21-24).
The aims of the present study were: first, to evaluate the aerobic cecal flora in cirrhotic rats and its relationship with BT; second, to establish the role of IBO in bacterial translocation; and third, to study intestinal motility in experimental cirrhosis.
MATERIAL AND METHODS
Twenty-seven rats with carbon tetrachloride-induced cirrhosis and ten normal rats were studied. The induction of cirrhosis was performed according to the experimental model described by Runyon et al. (25). Male Sprague-Dawley rats were caged under constant temperature and humidity conditions, with a 16/8 hour light/dark cycle, and fed with standard rodent chow, all in agreement with the guidelines for animal research in the Guide for the Care and Use of Laboratory Animals. Rats were observed daily. Cirrhosis induction began with the animals weighing 100-150 g, and by administering 1.5 mmol/l of phenobarbital in the drinking water. When the rats reached 200 g of body weight, intragastric CCl4 was given using a 50% dilution with olive oil by esophagic cannulation with a special needle and no anesthesia. CCl4 was given weekly with a starting dose of 40 µl, with subsequent doses being increased depending on the change in body weight.
Bacterial translocation and IBO study
When the animals reached an established cirrhotic situation (between 9 and 11 weeks) various samples were obtained for later processing. Anesthesia was induced by inhalation of ethyl ether (Iqanalítica, Probus S.A. Barcelona, Spain). One sample of peripheral blood was taken before laparotomy.
Laparotomy was then carried out under aseptic conditions, following removal of the abdominal fur and applying a local antiseptic solution. The peritoneum was then opened and potential sources of infection were searched for. Samples were obtained in the following order: portal blood, mesenteric lymph nodes from the ileo-cecal area, liver, and spleen; in all cases, this was performed before death. Finally, cecal samples were collected. Sections of the liver were also obtained for histological analysis.
Samples of peripheral and portal blood were cultured by immediate inoculation into blood culture bottles (Bactec Plus/ aerobio) and by later processing in a Bactec 9240 system (Becton-Dickinson. Maryland. USA). Liver, spleen, and MLN samples were homogenized in brain-heart medium and then plated (0.1 ml) on CNA and MacConkey agar for twenty-four hours. Blood bottles with a positive culture were plated in the same way. Cecal samples were homogenized in brain-heart medium, and diluted to 10-2, 10-4 and 10-6. Diluted homogenates of cecal samples were cultured (0.1 ml) in the same plates (CNA, and MacConkey). Microorganisms were identified by usual bacteriological methods: API 20E (Bio Mérieux SA. Mercy l'Etolile. France) and MicroScan (Baxter Healthcare, West Sacramento, CA, USA).
Bacterial translocation was defined as culture-positivity of MLN. Intestinal aerobic bacterial overgrowth was defined as a cecal bacterial count higher than the mean value plus two standard deviations of bacterial count in normal rats.
Intestinal transit determination
Gavage feedings of nonabsorbable liquid markers were given via an orogastric tube to determine intestinal transit. One hour before the BT study, 0.1 ml of Evans Blue solution (50 mg in 1 ml of 0.9% NaCl, Sigma Chemical Co.) was administered via the orogastric tube. Upon completion of the BT study, the whole small intestine was evaluated. As previously reported (26), intestinal transit was determined by measuring the distance between the gastric pylorus and the distal site of small intestine that was colored in blue.
Statistical analysis
Results are expressed as means or proportions, as required. Stools and MLN counts of colonies are presented as log10 colony-forming units (CFU) per ml of homogenate. Intestinal transit is expressed as the distance in centimeters between the gastric pylorus and the distal site of small bowel that was colored in blue. Kruskal-Wallis non-parametric ANOVA test was used for multiple comparisons. Mann-Whitney U test and Chi square or Fisher's exact test were used for comparisons between groups. A p-value of less than 0.05 was considered significant.
RESULTS
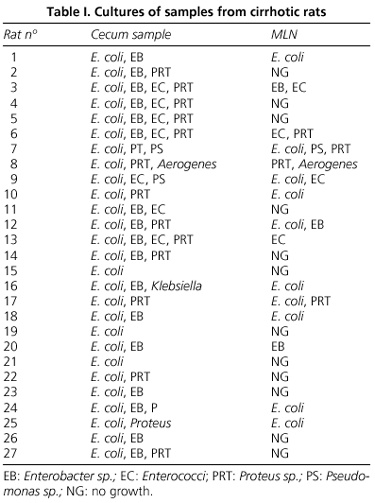
All the rats had cirrhosis at the time of the study. Regarding BT, pathogenic bacteria were cultured from the MLN of 56% of rats. None of the control rats translocated (p < 0.02). The majority of bacteria isolated were enteric gram-negative organisms, and the most frequent cultured organism was E. coli, which grew in 66.7% of cultures. Seven polymicrobial cases of BT were found. Total MLN bacterial count in translocated rats was 3.37 ± 1.24. Table I shows bacteria isolated in cecal samples and mesenteric lymph nodes.
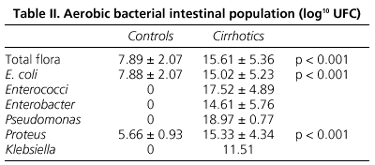
Total cecal aerobic bacterial CFU was significantly higher in cirrhotic rats (15.61 ± 5.36 log10 CFU) than in control rats (7.89 ± 2.07 log10 CFU) (p < 0.001). This increase of cecal bacterial count was mainly due to a greater UCF count of Escherichia coli (p < 0.001) and Proteus sp. (p < 0.001) (Table II). Enterococci were cultured in 7 cirrhotic rats and Enterobacter sp. in 17 cirrhotic animals, while they were not detected in control rats.
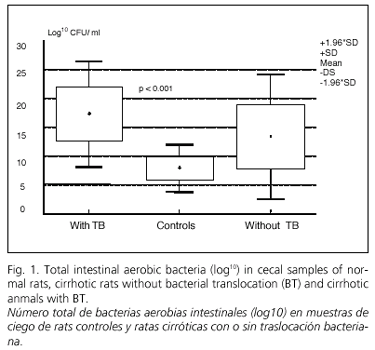
Cirrhotic rats were classified according to the presence or absence of BT; in 12 animals bacterial translocation was not detected. Total aerobic cecal bacterial counts in both groups and in controls are illustrated in figure 1. Cirrhotic rats with translocated bacteria had higher total aerobic intestinal counts (17.36 ± 4.71 log10 CFU) compared to culture-negative MLN bacteria (13.39 ± 5.52 log10 CFU) (p < 0.05). Cecal bacterial count was also higher in animals without BT than in controls (7.89 ± 2.07 log10 CFU). Significant differences in bacterial count were observed between groups (KW = 17.32, p < 0.001).
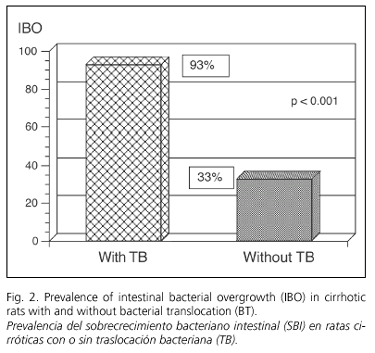
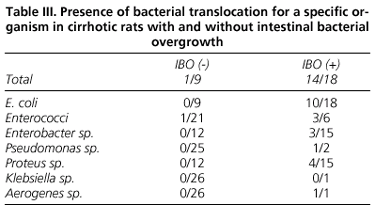
The prevalence of total intestinal bacterial overgrowth in cirrhotic animals was 67%. According to BT, total IBO was more frequent in cirrhotic rats with BT than in those without BT (93 vs. 33%) (p < 0.001) (Fig. 2). The relationship between translocation of a specific organism and the presence of intestinal overgrowth for this microorganism in cirrhotic rats was evaluated (Table III). Bacterial translocation of a specific bacterium was almost always associated with the IBO for that organism, and this occurred in all but one case of BT (only one enterococcus translocated without enterococci overgrowth in the cecum, although it was present in cecal cultures). Cecal overgrowth of one specific organism was not always associated with the translocation of that organism, and each organism showed a different translocation rate: E. coli 56%, Enterococci 50%, Pseudomonas sp. 50%, Proteus sp. 27%, Enterobacter sp. 20%. One animal presented cecal overgrowth and translocation to MLN regarding Aerogenes sp. (Table III).
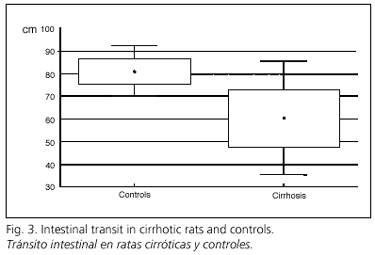
Intestinal transit was delayed in cirrhotic animals; the nonabsorbable marker travelled 60.5 ± 12.7 cm distal to the pylorus in cirrhotic rats, versus 81.2 ± 5.7 cm in controls (p < 0.05) (Fig. 3). It was not possible to establish a significant relationship between BT and intestinal transit time.
DISCUSSION
The passage of gut flora to MLN (bacterial translocation) has been postulated as the main pathogenic mechanism in cases with infection due to enteric gram-negative bacteria (1,27). Infectious complications by enteric flora are common in the setting of cirrhosis, and BT has been demonstrated to play an important role in the development of spontaneous bacteremia and spontaneous bacterial peritonitis (28-32).
This study confirms the high prevalence of BT in experimental cirrhosis, even in the early stages of the disease without ascites. In previous studies the development of BT was more frequent in cirrhotic rats with ascites, and García-Tsao found BT only in cirrhotic animals with ascites (4,6). Our data suggest that ascites is not necessary for the development of BT, and there must be other promoting factors in cirrhosis. There may be several causes for this high prevalence of BT in cirrhosis, such as intestinal mucosal abnormalities, associated malnutrition, immunological alterations, and intestinal bacterial overgrowth (4,33-39).
In the present study cirrhotic rats showed a significantly higher total intestinal aerobic bacteria count than control animals, mainly due to gram-negative enterobacteria (E. coli and Proteus). This moved the rate of IBO up to 67% in cirrhotic animals, which is in agreement with findings by other author (39,40). These bacteria are the most frequently implicated in the development of BT, as previously published (7,10,30,39). Moreover, we observed that the intestinal bacterial count in cirrhotic rats was higher in animals with BT than in those without it. Similarly, cirrhotic rats with IBO showed a higher rate of BT than those without IBO.
Such a relationship between IBO, BT, and the occurrence of infection has been seen as much at the experimental level as in human studies. In animal models IBO leads to translocation, and in cirrhotic animals it also leads to the development of SBP (6,7,41). Our results stand for the presumed role of bacterial overgrowth as a promoting factor of bacterial translocation in liver cirrhosis, then favoring the development of SBP. In fact, our group has previously found that in cirrhotic patients the presence of IBO was related to a higher incidence of SBP during hospitalization (13). All these data suggest that in liver cirrhosis intestinal bacterial overgrowth promotes BT and then the development of infectious complications such as SBP.
A point to highlight in our study is that even when translocation of a specific bacterium from the gut was associated with a cecal overgrowth of that same organism, not all animals with IBO showed translocation. Thus, in experimental cirrhosis, IBO for each bacteria is a necessary but not sufficient factor for the translocation of that organism, and therefore some other factors must be involved. Intrinsic virulence of bacteria might be one of these factors; there are microorganisms that are more predisposed to translocate, perhaps because of differences in its ability to penetrate the intestinal epithelium (42-44). Indeed, in our study we observed that the translocation rate of overgrown bacteria was different -E. coli and Enterococci were more predisposed, which is consistent with published results.
The indigenous gut flora is controlled by different defensive physiological mechanisms, such as gastrointestinal motility, gastric acidity, and bacterial interactions (36,42,45-47). Some of these can be impaired in cirrhosis, where hypoclorhidria (15) and delayed small intestinal transit (47-51) have been described. Evidence from various studies has shown that altered intestinal motility may be involved in the pathogenesis of IBO in patients with liver disease (23,51), and could be associated with a higher incidence of SBP (24). A number of studies where the administration of prokinetic drugs caused a decrease in both IBO and BT point at the contribution of altered intestinal motility to these phenomena. In our study, cirrhotic animals showed a slower intestinal transit than controls, although a significant relationship between this dismotility and BT could not be established. The lack of this statistic correlation may be due to the non-direct nature of the method used to assess intestinal transit compared with the more precise methods used in other studies (55,56).
Taken together, these data suggest that intestinal bacterial overgrowth in the setting of cirrhosis may appear early, yet in the absence of ascites, and it is one of the factors triggering bacterial translocation. In addition, it seems that overgrowth of a specific bacteria is a necessary but non-sufficient condition to produce translocation. Finally, cirrhosis is associated with reduced intestinal motility, which prevents normal peristaltic clearance; this may result in enteric bacterial overgrowth and subsequent translocation.
REFERENCES
1. Alexander JW, Boyce ST, Babcock GF, Gianotti L, Peck MD, Dunn DL, et al. The process of microbial translocation. Ann Surg 1990; 212: 496-512. [ Links ]
2. Mainous MR, Tso P, Berg RD, Deitch MD. Studies of the route, magnitude, and time course of bacterial translocation in a model of systemic inflammation. Arch Surg 1991; 126: 33-7. [ Links ]
3. Rimola A, Soto R, Bory F, Arroyo V, Piera C, Rodes J. Reticuloendothelial system phagocytic activity in cirrhosis and its relation to bacterial infections and prognosis. Hepatology 1984; 4: 53-8. [ Links ]
4. Garc'a-Tsao G, Lee FY, Barden GE, Cartun R, West AB. Bacterial translocation to mesenteric lymph nodes is increased in cirrhotic rats with ascites. Gastroenterology 1995; 108: 1835-41. [ Links ]
5. Llovet JM, Bartol' R, Planas R, Cabre E, Jiménez M, Urban A, et al. Bacterial translocation in cirrhotic rats. Its role in the development of spontaneous bacterial peritonitis. Gut 1994; 35: 1648-52. [ Links ]
6. Runyon BA, Squier S, Borzio M. Translocation of gut bacteria in rats with cirrhosis to mesenteric lymph nodes partially explains the pathogenesis of spontaneous bacterial peritonitis. J Hepatol 1994; 21: 792-6. [ Links ]
7. Martín L, Sánchez E, Casafont F, Agüero J, Pons Romero F. Desarrollo de peritonitis bacteriana espontánea en un modelo experimental de rata cirrótica. Relación con la traslocación bacteriana intestinal. Rev Esp Enferm Dig 1995; 87: 632-6. [ Links ]
8. García-Tsao G, Albillos A, Barden GE, West AB. Bacterial translocation in acute and chronic portal hypertension. Hepatology 1993; 17: 1081-5. [ Links ]
9. Deitch EA, Xu DZ, Oi L, Specian RD, Berg RD. Protein malnutrition alone and in combination with endotoxin impairs systemic and gut-associated immunity. JPEN 1992; 16: 25-31. [ Links ]
10. Casafont F, Sánchez E, Martín L, Agüero J, Pons Romero F. Influence of malnutrition on the prevalence of bacterial translocation and spontaneous bacterial peritonitis in experimental cirrhosis. Hepatology 1997; 25: 1334-7. [ Links ]
11. Wells CL. Colonization and translocation of intestinal bacterial flora. Transplant-Proc 1996; 28 (5): 2653-6. [ Links ]
12. O'Boyle CJ, MacFie J, Mitchell CJ, Johnstone D, Sagar PM, Sedman PC. Microbiology of bacterial translocation in humans. Gut 1998; 42 (1): 29-35. [ Links ]
13. Morencos FC, De las Heras G, Martín Ramos L, Lopez MJ, Pons Romero F. Small bowel bacterial overgrowth in patients with alcoholic cirrhosis. Dig Dis Sci 1995; 40: 1252-6. [ Links ]
14. Yang CY, Chang CS, Chen GH. Small-intestine bacterial overgrowth in patients with liver cirrhosis, diagnosed with H2 and CH4 breath tests. Scand J Gastroenterol 1998; 33: 867-71. [ Links ]
15. Chesta J, Silva M, Thompson L, del Canto E, Defilippi C. Sobrecrecimiento bacteriano del intestino delgado en pacientes con cirrosis hepática. Rev Med Chile 1991; 119: 626-32. [ Links ]
16. Bode Ch, Kolepke R, Schäfer K, Bode J Ch. Breath hydrogen excretion in patients with alcoholic liver disease - evidence of small intestinal bacterial overgrowth. Z. Gastroenterol 1993; 31: 3-7. [ Links ]
17. Gines P, Rimola A, Planas R, Vargas V, Marco F, Almela M, et al. Norfloxacin prevents spontaneuos bacterial peritonitis recurrence in cirrhosis: results of a double-blind, placebo-controlled trial. Hepatology 1990; 12: 716-24. [ Links ]
18. Runyon BA, Borzio M, Young S, Squier SU, Guarner C, Runyon MA. Effect of selective bowel decontamination with norfloxacin on spontaneous bacterial peritonitis, translocation, and survival in an animal model of cirrhosis. Hepatology 1995; 21: 1719-24. [ Links ]
19. Llovet JM, Rodríguez-Iglesias P, Moitinho E, Planas R, Bataller R, Navasa M, et al. Spontaneous bacterial peritonitis in patients with cirrhosis undergoing selective intestinal decontamination. A retrospective study of 229 spontaneous bacterial peritonitis episodes. J Hepatol 1997; 26 (1): 88-95. [ Links ]
20. Soriano G, Guarner C, Teixido M, Such J, Barrios J, Enríquez J, et al. Selective inestinal decontamination prevents spontaneous bacterial peritonitis. Gastroenterology 1991; 100: 477-81. [ Links ]
21. Wang XD, Soltesz V, Andersson R. Cisapride prevents enteric bacterial overgrowth and translocation by improvement of intestinal motility in rats with acute liver failure. Eur Surg Res 1996; 28: 402-12. [ Links ]
22. Runkel NS, Moody FG, Smith GS, Rodríguez LF, Chen Y, Larocco MT, et al. Alterations in rat intestinal transit by morphine promote bacterial translocation. Dig Dis Sci 1993; 38: 1530-6. [ Links ]
23. Chesta J, Defilippi C, Defilippi C. Abnormalities in proximal small bowel motility in patients with cirrhosis. Hepatology 1993; 17: 828-32. [ Links ]
24. Chang CS, Chen GH, Lien HC, Yeh HZ. Small intestine dysmotility and bacterial overgrowth in cirrhotic patients with spontaneous bacterial peritonitis. Hepatology 1998; 28: 1187-90. [ Links ]
25. Runyon BA, Sugano S, Danel G, Mellencamp M. A rodent model of cirrhosis and spontaneous bacterial peritonitis. Gastroenterology 1991; 100: 489-93. [ Links ]
26. Wirthlin DJ, Cullen JJ, Spates ST, Conklin JL, Murray J, Caropreso DK, et al. Gastrointestinal transit during endotoxemia: the role of nitric oxide. J Surg Res 1996; 60: 307-11. [ Links ]
27. Baron P, Traber LD, Traber DL, Nguyen T, Hollyoak M, Heggers JP, et al. Gut failure and translocation following burn and sepsis. J Surg Res 1994; 57 (1): 197-204. [ Links ]
28. Navasa M, Rimola A, Rodes J, Bacterial infections in liver disease. Seminars Liver Dis 1997; 17: 323-33. [ Links ]
29. Caly WR, Strauss E. A prospective study of bacterial infections in patients with cirrhosis. J Hepatol 1993; 18: 353-8. [ Links ]
30. Llovet JM, Bartol' R, Planas R, Cabré E, Jiménez M, Urban A, et al. Bacterial translocation in cirrhotic rats. Its role in the development of spontaneous bacterial peritonitis. Gut 1994; 35: 1648-52. [ Links ]
31. Guarner C, Soriano G. Spontaneous bacterial peritonitis. Seminars Liver Dis 1997; 17: 203-17. [ Links ]
32. Runyon BA. Bacterial infections in patients with cirrhosis. J Hepatol 1993; 18: 271-2. [ Links ]
33. Garcia-Tsao G. Spontaneuos bacterial peritonitis. Gastroenterol Clin North Am 1991; 21: 257-75. [ Links ]
34. Sorell WT, Quigley EMM, Jin G, Johnson TJ, Rikkers LF. Bacterial translocation in the portal-hypertensive rat: studies in basal conditions and on exposure to hemorrhagic shock. Gastroenterology 1993; 104: 1722-6. [ Links ]
35. Pelletier G, Briantais MJ, Buffet C, Pillot J, EtiennePelletier G, Briantais MJ, et al. Serum and intestinal secretory Ig A in alcohlic cirrhosis of the liver. Gut 1982; 23: 475-80. [ Links ]
36. Kirsch M. Bacterial overgrowth. Am J Gastroenterol 1990; 85: 231-7. [ Links ]
37. Steffen E, Berg R. Relationship between caecal population levels of indigenous bacteria and translocation to mesenteric lymph nodes. Infect Inmmun 1983; 39: 1252-9. [ Links ]
38. O'Boyle CJ, MacFie J, Mitchell CJ, Johnstone D, Sagar PM, Sedman PC. Microbiology of bacterial translocation in humans. Gut 1998; 42 (1): 29-35. [ Links ]
39. Guarner C, Runyon BA, Young S, Heck M, Sheikh MY. Intestinal bacterial overgrowth and bacterial translocation in cirrhotic rats with ascites. J Hepatol 1997; 26: 1372-8. [ Links ]
40. Llovet JM, Bartoli R, March F, Planas R, Vinado B, Cabré E, et al. Translocated intestinal bacteria cause spontaneous bacterial peritonitis in cirrhotic rats: molecular epidemiologic evidence. J Hepatol 1998; 28: 307-13. [ Links ]
41. Berg RD. Bacterial translocation from the gastrointestinal tract. J Med 1992; 23 (3-4): 217-44. [ Links ]
42. Wells CL, Jechorek RP, Gillingham KJ. Relative contributions of host and microbial factors in bacterial translocation. Arch Surg 1991; 126: 247-52. [ Links ]
43. Katouli M, Bark T, Ljungqvist O, Svenberg T, Mollby R. Composition and diversity of intestinal coliform flora influence bacterial translocation in rats after hemorrhagic stress. Infect Immun 1994; 62 (11): 4768-74. [ Links ]
44. Steffen E, Berg R, Deitch E. Comparison of translocation rates of various indigenous bacteria from the gastrointestinal tract to the mesenteric lymph nodes. J Infect Dis 1988; 157: 1032-8. [ Links ]
45. Drude RB, Hines C. The pathophysiology of intestinal bacterial overgrowth syndromes. Arch Intern Med 1980; 140: 1349-52. [ Links ]
46. Wells CL, Maddaus MA, Reynolds CM, Jechorek RP, Simmons RL. Role of anaerobic flora in the translocation of aerobic and facultatively anaerobic intestinal bacteria. Infect Immun 1987; 55: 2689-94. [ Links ]
47. Dinsmore JE, Jackson RJ, Smith SD. The protective role of gastric acidity in neonatal bacterial translocation. J Pediatr Surg 1997; 32 (7): 1014-6. [ Links ]
48. Madrid AM, Brahm J, Buckel E, Silva G, Defilippi C. Orthotopic liver transplantation improves small bowel motility disorders in cirrhotic patients. Am J Gastroenterol 1997; 92: 1044-5. [ Links ]
49. Husebye E. Gastrointestinal motility disorders and bacterial overgrowth. J Intern Med 1995: 237: 419-27. [ Links ]
50. Chesta J, DeFilippi C, DeFilippi C. Abnormalities in proximal small bowel motility in patients with cirrhosis. Hepatology 1993; 17: 828-32. [ Links ]
51. Van Thiel DH, Fagiuoli S, Wright HI, Chien MC, Gavaler JS. Gastrointestinal transit in cirrhotic patients: effect of hepatic encephalopathy and its treatment. Hepatology 1994; 19: 67-71. [ Links ]
52. Pardo A, Bartoli R, Lorenzo-Zuniga V, Planas R, Vinado B, Riba J, et al. Effect of cisapride on intestinal bacterial overgrowth and bacterial translocation in cirrhosis. Hepatology 2003; 31: 858-63. [ Links ]
53. Pérez-Paramo M, Muñoz J, Albillos A, Freile I, Portero F, Santos M, et al. Effect of propranolol on the factors promoting bacterial translocation in cirrhotic rats with ascites. Hepatology 2003; 31: 43-8. [ Links ]
54. Zhang SC, Wang W, Ren WY, He BM, Zhou K, Zhu WN. Effect of cisapride on intestinal bacterial and endotoxin translocation in cirrhosis. World J Gastroenterol 2003; 9: 534-8. [ Links ]
55. Reilly JA, Quigley EMM, Forst CF, Rikkers LF. Small intestinal transit in the portal hypertensive rat. Gastroenterology 1991; 100: 670-4. [ Links ]
56. Chesta J, Debnam ES, Srai SKS, Epstein O. Delayed stomach to caecum transit time in the diabetic rat. Possible role of hyperglucagonemia. Gut 1990; 30: 660-2. [ Links ]











 texto em
texto em