Meu SciELO
Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Revista Española de Enfermedades Digestivas
versão impressa ISSN 1130-0108
Rev. esp. enferm. dig. vol.103 no.3 Madrid Mar. 2011
Prophylaxis and treatment of hepatitis B infection in the setting of liver transplantation
Profilaxis y tratamiento de la infección por el virus de la hepatitis B en relación con el trasplante hepático
Delia D'Avola and José Ignacio Herrero
Liver Unit. Clinica Universitaria. Pamplona, Navarra. Spain and centro de Investigación Biomédica en Red de enfermedades hepáticas y digestivas (CIBERehd)
ABSTRACT
Without any treatment, the prognosis of hepatitis B in liver transplant recipients is very poor. So, antiviral prophylaxis is very important in patients with hepatitis B who undergo liver transplantation. Before liver transplantation, a suppression of viral replication has to be achieved by nucleos(t)ide analogs. Drugs used in the prophylaxis of post-transplant hepatitis B include immunoglobulin against HBV and nucleos(t)ide analogs. Prophylaxis against graft infection must be based on the individual risk of recurrence. When prophylactic measures have failed and graft infection has occurred, treatment of recurrent hepatitis B may be based on the resistance profile of the virus and previous antiviral exposure. Finally, lamivudine seems to be very effective in the prevention of de novo hepatitis B in patients transplanted with a graft from an anti-HBc positive donor.
Key words: Hepatitis B. Prophylaxis. Liver transplantation.
RESUMEN
La infección por el virus de la hepatitis B en los receptores de trasplante hepático tiene un pronóstico malo en ausencia de tratamiento farmacológico. Por ello, en los pacientes trasplantados por una hepatopatía por virus B, la profilaxis de esta infección es imprescindible. Antes del trasplante, debe intentarse suprimir la replicación viral con un análogo de nucleót(s)idos. Tras el trasplante, la profilaxis de la recidiva de la hepatitis B se basa en el uso de los análogos de nucleót(s)idos y la inmunoglobulina frente al virus de la hepatitis B; la pauta de profilaxis debe basarse en el riesgo de recidiva de la hepatitis B que tenga cada paciente. En el caso de que se produzca una hepatitis B tras el trasplante, el tratamiento con análogos de nucleót(s)idos debe basarse en los estudios de resistencia. Por último, en los pacientes trasplantados que reciben un injerto de un donante antiHBc-positivo, la profilaxis con lamivudina parece ser muy eficaz.
Palabras clave: Hepatitis B. Profilaxis. Trasplante hepático.
Abbreviations
HBV: hepatitis B virus
DNA: dexosiribonucleic acid
HDV: hepatitis D virus
Anti-HBs: antibodies against HBV surface antigen
HBeAg: antibodies against HBV e antigen
anti-HBc: antibodies against core antigen
MELD: Model for End-stage Liver Disease
Introduction
Hepatitis B virus (HBV) related liver cirrhosis accounts for 5-10% of liver transplantations performed in Spain (1). Until latest '80s prognosis of transplant recipients for HBV-related cirrhosis was very poor, and graft loss due to recurrent HBV infection was the rule. In the last two decades, the availability of hyperimmune immunoglobulin and oral antiviral agents, have definitely changed the prognosis of liver transplantation for HBV-related diseases. Currently, 5-years survival rate is around 85%, compared to 45% at 3 years before the immunoglobulin era (1,2).
Hepatitis B recurrence still plays an important role in determining survival after liver transplantation, and in some series it has been suggested that recurrent hepatitis B may have a role in promoting recurrence of liver cancer (3) and lymphoproliferative disorders after transplantation (4). Antiviral treatment, before and after liver transplantation, markedly reduces the risk of hepatitis B recurrence. High levels of HBV-DNA and presence of HBV e antigen (HBeAg) at the time of liver transplantation, as well as HBV-related liver cirrhosis are the most important predictors of hepatitis B recurrence (5,6). Conversely, acute liver failure and HBV-HDV coinfection have been regarded as protective factors.
In the early '90, a multicenter European study showed a 32% recurrence rate among HBV-hepatitis D virus (HDV) coinfected patients compared to 53% in patients with HBV infection alone (6). The protective role of HDV infection has been shown only in liver cirrhosis, but not in acute hepatitis B, and it is supposed to be related to the inhibition of HBV replication (7).
In the setting of liver transplantation, three different clinical situations have to be distinguished: a) treatment of HBV infection during waiting list; b) prophylaxis of hepatitis B recurrence after liver transplantation; and c) treatment of recurrent hepatitis B when prophylactic measures have failed. Moreover, de novo hepatitis B can occur when liver from donors with antibodies against the HBV core antigen (anti-HBc) are transplanted. This donors account for 10-12% of the livers transplanted in Spain (1).
Treatment of hepatitis B prior to liver transplantation
The availability of oral antiviral drugs, in the latest decades, has definitely changed the management of HBV-liver cirrhosis. Lamivudine, the first oral antiviral agent against HBV, is safe and well tolerated even in patients with decompensated liver cirrhosis (8). Patients with decompensated HBV-liver cirrhosis may benefit of lamivudine treatment: their liver function may improve, complications of liver cirrhosis may resolve and, in some cases, liver transplantation is not longer needed (9-11). Hyperimmune immunoglobulin and nucleos(t)ide analogs, administered after liver transplantation, dramatically reduce the risk of hepatitis B recurrence; however, patients with a high HBV-viral load at the time of liver transplantation have a high risk of recurrent hepatitis, despite prophylaxis (6,12).
For this reason, the main national and international hepatology societies (Asociación Española para el Estudio del Hígado, AEEH, American Association for the Study of Liver Diseases, AASLD, and European Association for the Study of the Liver, EASL) recommend the aggressive treatment of HBV infection before liver transplantation (13-15).
The goal of antiviral therapy during waiting list is double: first, to minimize viral replication prior to transplantation (ideally achieving a total suppression of viral replication), and secondly, to improve liver function in order to reduce mortality during waiting list (16). In the setting of liver transplantation, as well as in other clinical situations, the choice of antiviral drugs depends on the severity of liver cirrhosis. Patients with chronic hepatitis or compensated liver cirrhosis, that usually have hepatocellular carcinoma, should be treated equally to patients not listed for liver transplantation. Highly potent antiviral agents (e.g. entecavir, tenofovir or telbivudine) are recommended as monotherapy or as combination therapy, according to the presence of comorbidities and the resistance profile of HBV. However, safety and efficacy data of these new antiviral agents are not yet available. As a consequence, lamivudine and adefovir are the only two agents approved for the treatment of decompensated liver cirrhosis and lamivudine is the most used.
A major concern of long-term lamivudine therapy is the high resistance rate that reaches 10-20% at 1 year and is above 60% after 4 years of treatment. Although the long-term resistance rate should not be an issue in a waiting list setting, a 10% of lamivudine-resistance rate has been reported among a series of wait-listed patients, because of the emergence of resistant strains after 1 year of treatment (9,11,17). In addition, the duration of waiting time is not predictable and the HBV reactivation in cirrhotics could result in high mortality and morbidity (18). So, although it is approved for HBV decompensated liver cirrhosis, lamivudine alone is not longer recommended in this setting and its association with adefovir is strongly suggested (14).
Adefovir is effective for lamivudine-resistant strains, resulting in the suppression of viral replication. Adefovir has been successfully used in patients with advanced cirrhosis and lamivudine-resistant hepatitis B, leading to an improvement of liver function as shown by the reduction of Child-Pugh score (19). The main concern about adefovir treatment is the risk or kidney dysfunction. In addition, compared to other antiviral agents, the time to achieve a complete viral suppression with adefovir could be longer (20,21).
New oral antiviral agents seem a promising possibility for the future in the treatment of wait-listed patients, however they are not already approved for the use in decompensated liver cirrhosis. Among these, entecavir is highly and rapidly effective, and it could be used when a rapid viral suppression is required or in patients showing neprotoxicity related to adefovir. It has been recently showed highly effective on inhibiting viral replication among cirrhotic patients. However its safety among patients with poor liver function remain uncertain, because of some reports of lactic acidosis in severely decompensated liver disease (MELD > 18) (22,23).
Compared to entecavir, tenofovir is more effective among lamivudine-resistant strains, and it has a better therapeutic index in patients with impaired renal function, compared to adefovir (24).
Because of their efficacy and safety, entecavir and tenofovir, used as monotherapy or in combination, are considered as the first choice agents, in patients with chronic hepatitis or compensated liver cirrhosis listed for liver transplantation.
Finally, telbivudine has shown powerful antiviral activity, with lower resistance rates compared to lamivudine. One of the major advantages of telbivudine is its activity against adefovir-resistant strains. It is approved for use in compensated liver disease but, safety data in patients with decompensated liver disease are not available to date (21).
While treatment of chronic liver disease during waiting list is universally accepted, there is not a general consensus about treatment of patients with acute liver failure who are listed for liver transplantation. As mentioned before, the risk of recurrent hepatitis B in patients transplanted for acute hepatitis B is lower than that observed in patients transplanted for liver cirrhosis. However some recent reports suggest a possible role of antiviral agents in improving the prognosis of acute liver failure and its use in this setting is currently debated (6,25,26).
Summarizing, in patients in waiting list for liver transplantation, the choice of antiviral agent should take into account the degree of liver dysfunction. For patients with compensated liver disease (i.e., compensated liver cirrhosis or chronic hepatitis) a high potency antiviral agent, such as entecavir, tenofovir and telbivudine, is strongly recommended, according to comorbidities and the resistance profile.
In patients with decompensated liver cirrhosis, the association of lamivudine and adefovir is considered the first line therapy, at least in naïve patients not requiring an immediate viral suppression. Newer antiviral agents, not already approved for decompensated liver cirrhosis, are an attractive option for patients listed for liver transplantation, especially when a rapid viral suppression is needed, in presence of resistance to lamivudine or adefovir or in patients with contraindications to these two agents.
Prophylaxis of hepatitis B recurrence after liver transplantation
Ideally, the treatment of patients on the waiting list achieves a complete suppression of viral replication at the time of liver transplantation. Pre-transplant treatment reduces the recurrence rate in the graft (6,12). Nevertheless, to prevent hepatitis B recurrence, prophylactic measures have to be taken immediately after liver transplantation.
Hyperimmune immunoglobulin against HBV has been the first effective treatment in the prophylaxis of hepatitis B recurrence after liver transplantation. Nowadays, immunoglobulin are the most used drug in this clinical setting; however there is no a general agreement on dose, administration route and treatment schedules that have to be used (27,28).
Unfortunately, the use of immunoglobulin alone, even with long-term administration, results in 36 and 56% of recurrence rate at 3 and 5-years after liver transplantation, at least among patients with high recurrence risk (i.e. patients with positive HBV-DNA at the time of transplantation, or in patients with HBeAg) (6).
The efficacy of lamivudine as monotherapy is similar to that of immunoglobulin alone, leading to a 40-50% of recurrence at 3-years after transplantation (29,30). The combination of both has emerged as the most effective prophylactic strategy in HBV transplant recipients, resulting in a 3-years recurrence rate of 0-10% in the majority of published series (31-33).
The major concerns with the immunoglobulin therapy are the high cost and the administration route (parenteral), especially when considering the need of long term prophylaxis. Consequently, in the last 10 years, alternative therapeutic regimens with low dose immunoglobulin or using different antiviral agents have been evaluated.
In the attempt to lowering costs without decreasing the efficacy, combination of lamivudine with intramuscular immunoglobulin and/or therapeutic protocols including low dose immunoglobulin regimens (400-800 UI/months) have been investigated (34,35). Similarly, other investigators have explored individualized immunoglobulin dosing, according to anti-hepatitis B surface (anti-HBs) titers. This strategy could reduce the pharmacy cost of antiviral prophylaxis but increases the complexity of the follow-up of the patient and the work load for nurses and physician. Currently the global recurrence rate with the different proposed therapeutic protocols, including the association of nucleos(t)ide analogs and immunoglobulin, is reported around 0 and 10%, varying accordingly to the different risk factors at the time of liver transplantation (34,36-38). When considering patients with lamivudine-resistant hepatitis B, prophylactic treatment with immunoglobulin and lamivudine are less effective, resulting in recurrence rates of 15-22% (39). In these patients the use of a different antiviral agent (according to the resistance profile) in combination with immunoglobulin, should be considered, and the treatment choice has to regard the resistance profile. In this clinical setting, adefovir is probably the most explored antiviral agent. When it is used in patients with lamivudine resistance, the recurrence rate is 10% or lower (19).
The withdrawal of immunoglobulin, while long term oral antiviral treatment, is a safe therapeutic strategy, at least in patients with low risk of recurrence or in patients without recurrence after a prolonged treatment with combined lamivudine and immunoglobulin (40-45). In low-risk patients, who were HBV-negative at the time of liver transplantation, and received long term prophylaxis with lamivudine, the recurrence rates were similar when they received immunogloblulins for a short period of weeks or indefinitely (40). The effect of early withdrawn of immunoglobulin, while on long-term lamivudine prophylaxis, leads to 8-18% recurrence. Compliance with oral antivirals is critical for long-term outcome of this strategy (41).
Among low-risk patients after 12 months of immunoglobulin prophylaxis, other antiviral agents, such as adefovir, have been investigated as an alternative to long-term immunoglobulin (36). Similarly, newer high potency antivirals with a lower long-term resistance rate, are a promising option in an attempt to reduce the duration of immunoglobulin prophylaxis or to use low-dose regimens (46).
Active immunization with recombinant HBV vaccines has been investigated as an alternative to long-term immunoglobulin in low-risk patients (47-49). Most patients included in these studies had undetectable HBV replication and HBeAg-negative at the time of liver transplantation, did not show recurrent hepatitis B after the withdrawal of immunoglobulin prophylaxis and had been transplanted many years before, so they required a medium-low grade of immunosuppresion. Nevertheless, the percentage of patients achieving protective anti-HBs titers varies between the different reports, ranging from 82% reported in the Spanish series to 18% of the Italian series (49,50). In the same way, the efficacy and the persistency of protective titers have not been determined among large series, thus the use of these therapeutic regimens is not routinely recommended at present.
In conclusion, combined prophylaxis with hyperimmune immunoglobulin and lamivudine (that could be replaced by other antivirals according to the resistance profile), is the most effective therapeutic regimen to prevent hepatitis B recurrence after liver transplantation. However the duration of immunoglobulin treatment is still matter of debate.
Prophylactic strategies should be based in the risk level at the time of transplantation and the virological status after liver transplantation. In our opinion, high potency antivirals agents and long-term immunoglobulin should be employed for high risk patients, while for low and intermediate risk patients, less potent antivirals and early withdrawn immunoglobulin regimens should be considered (Fig. 1).
Liver recipients from anti-HBc-positive donors
Prevalence of antibodies against the HBV core antigen (anti-HBc) among liver donors varies according to the prevalence of hepatitis B in different geographic areas. It reaches 30-80% of liver donors in Asia and 10% in western countries (10-12% in Spain) (1,51, 52).
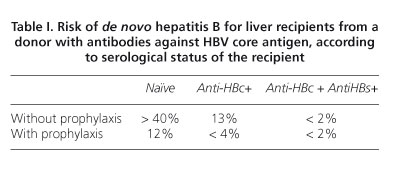
Anti-HBc-positivity of the donor is the main risk factor for de novo hepatitis B in liver transplant recipients. In older reports, patients who receive a graft form and anti-HBc- positive donor have shown a poor survival, but in the majority of recent series, survival among patients receiving an anti-HBc-positive graft is comparable to that of recipients of anti-HBc-negative liver (1,53,54). Serological status of the receptor and the viral load in the graft, strongly determine the risk of de novo hepatitis B among receptors of antiHBc-positive graft. Without any prophylaxis, the risk of de novo hepatitis in naïve patients receiving anti-HBc-positive graft is 40% compared to 10% in recipients with antibodies against HBV surface antigen (anti-HBs). This risk is similar (13%) in recipients who only have anti-HBc, and is very low (2%) in patients with both anti-HBc and anti-HBs (55) (Table I).
Among the several prophylactic regimens for the prevention of de novo hepatitis B in anti-HBc-positive graft recipients, long-term lamivudine is the most used.
Depending on the serological status of the recipient, patients receiving prophylaxis with lamivudine have a risk of developing de novo hepatitis B below 4% (53, 56,57). In some centers lamivudine is combined with immunoglobulin, however a real advantage of this strategy compared to lamivudine alone has never been shown (58,59).
Immunoglobulin prophylaxis is less effective than lamivudine, leading to a 10% of de novo hepatitis B among antiHBc-positive recipients and above 20% among seronegative patients (60-62).
Lamivudine prophylaxis is almost universally accepted and is strongly recommended in all liver recipients rgardless their serological status, even in anti-HBc-positive recipients. According to the low rate of de novo hepatitis in anti-HBc-positive recipients, prophylaxis could be unnecessary in them.
Treatment of hepatitis B in liver transplantation recipients
At the present time, recurrence of hepatitis B occurs in less than 20% of liver recipients 4 years after liver transplantation (2). Among transplanted patients, HBV infection (de novo hepatitis or recurrent) could show an aggressive clinical course, characterized by a marked inflammation and rapidly progressive fibrosis. Cholestasic fibrosing hepatitis is an exclusive hepatitis B pattern of immunosuppresed patients. It is characterized by lobular disarray, cholangiolar proliferation, severe fibrosis and mild inflammation (63-65). Treatment of hepatitis B among liver recipients is based on the same principles as in not transplanted patients. However, in presence of an aggressive clinical course, when a rapid suppression of viral replication is required, high potency antiviral agents should be used (66). As in non-transplanted patients, the choice of treatment must be based on the previous antiviral treatments, the resistance profile and the presence of comorbidities, especially renal insufficiency.
Until the early 2000, the most used antiviral agents against HBV have been interferon and lamivudine. Nowadays, because of its limited efficacy and the risk of graft rejection, interferon is not longer used in this clinical setting. Similarly, due to the need of long-term treatment and considering that lamivudine resistance rate raises the 70% after 4-5 years of treatment, lamivudine monotherapy is currently abandoned (67). The availability of adefovir has changed the clinical course of lamivudine-resistant recurrent hepatitis B, and it is effective and safe among liver transplantation recipients (19,68,69). However, adefovir treatment could lead to the emergence of resistant strains or to incomplete suppression of viral replication; moreover it is potentially nephrotoxic and its dosage must be adjusted in patients with impaired renal function.
Tenofovir is effective against lamivudine resistant strains and it is safe in the setting of liver transplantation. Due to its good therapeutic index and the lower resistance rate compared to adefovir, it could be a valuable therapeutic option at least for patients with kidney dysfunction (70,71). Telbivudine, has a possible role in the presence of adefovir resistant strains, however its safety and efficacy have not been proven in liver recipients.
Entecavir is highly effective in patients naïve for nucleos(t)ide analogs. However its antiviral activity is limited among patients with lamivudine resistant hepatitis B, in whom a genotypic resistance to entecavir has been found around 15% after 2 years of treatment (72). As the other newer antiviral agents, entecavir is an attractive option for liver receptors; however safety and efficacy data in this clinical setting are needed.
Conclusions
Antiviral treatment is strongly recommended among all candidates for liver transplantation with active hepatitis B infection. The ideal antiviral agent should have a minimal risk of resistance and achieve a rapid viral suppression. The goal of the treatment is to achieve a complete suppression of viral replication at the time of liver transplantation, in order to reduce the risk of recurrence after transplantation. In Spain, lamivudine and adefovir are the only two antiviral agents approved for patients with decompensated liver cirrhosis; however, in specific situations, the use of other agents, such as entecavir, tenofovir and telbivudine, should be considered accordingly to the presence of comorbidities or previous antiviral resistance.
Independently of the serological status of the recipients, after transplantation, prophylaxis should be given without delay. Immunoglobulin and one or two nucleot(s)ide analogs selected considering the virological status should be given. When prophylaxis has failed, hepatitis B recurrence has to be rapidly treated, according to the resistance profile and comorbidities, as in non-transplanted patients. Finally all HBsAg- negative patients receiving an HBcAb-positive graft have to receive long-term prophylaxis with lamivudine.
References
1. Sociedad Española de Trasplante Hepático y Organización Nacional de Trasplantes. Memoria de resultados 1984-2008. Registro Español de Trasplante Hepático, 2009. Available at: http://www.ont.es/infesp/registros/memoria_RETH_2009.pdf [ Links ]
2. Bzowej N, Han S, Degertekin B, Keeffe EB, Emre S, Brown R, et al. Liver transplantation outcomes among Caucasians, Asian Americans, and African Americans with hepatitis B. Liver Transpl 2009; 15:1010-20. [ Links ]
3. Saab S, Yeganeh M, Nguyen K, Durazo F, Han S, Yersiz H, et al. Recurrence of hepatocellular carcinoma and hepatitis B reinfection in hepatitis B surface antigen-positive patients after liver transplantation. Liver Transpl 2009;15:1525-34. [ Links ]
4. Zhang A, Zhang M, Shen Y, Wang W, Zheng S. Hepatitis B virus reactivation is a risk factor for development of post-transplant lymphoproliferative disease after liver transplantation. Clin Transplant 2009; 23:756-60. [ Links ]
5. Anselmo DM, Ghobrial RM, Jung LC, Weaver M, Cao C, Saab S, et al. New era of liver transplantation for hepatitis B: a 17-year single-center experience. Ann Surg 2002;235:611-9; discussion 619-20. [ Links ]
6. Samuel D, Muller R, Alexander G, Fassati L, Ducot B, Benhamou JP, et al. Liver transplantation in European patients with the hepatitis B surface antigen. N Engl J Med 1993;329:1842-7. [ Links ]
7. Marsman WA, Wiesner RH, Batts KP, Poterucha JJ, Porayko MK, Niesters HG, et al. Fulminant hepatitis B virus: recurrence after liver transplantation in two patients also infected with hepatitis delta virus. Hepatology 1997;25:434-8. [ Links ]
8. Villeneuve JP, Condreay LD, Willems B, Pomier-Layrargues G, Fenyves D, Bilodeau M, et al. Lamivudine treatment for decompensated cirrhosis resulting from chronic hepatitis B. Hepatology 2000; 31:207-10. [ Links ]
9. Yao FY, Terrault NA, Freise C, Maslow L, Bass NM. Lamivudine treatment is beneficial in patients with severely decompensated cirrhosis and actively replicating hepatitis B infection awaiting liver transplantation: a comparative study using a matched, untreated cohort. Hepatology 2001;34:411-6. [ Links ]
10. Perrillo RP, Wright T, Rakela J, Levy G, Schiff E, Gish R, et al. A multicenter United States-Canadian trial to assess lamivudine monotherapy before and after liver transplantation for chronic hepatitis B. Hepatology 2001;33:424-32. [ Links ]
11. Fontana RJ, Keeffe EB, Carey W, Fried M, Reddy R, Kowdley KV, et al. Effect of lamivudine treatment on survival of 309 North American patients awaiting liver transplantation for chronic hepatitis B. Liver Transpl 2002;8:433-9. [ Links ]
12. Marzano A, Gaia S, Ghisetti V, Carenzi S, Premoli A, Debernardi-Venon W, et al. Viral load at the time of liver transplantation and risk of hepatitis B virus recurrence. Liver Transpl 2005;11:402-9. [ Links ]
13. Consensus document of the Spanish Association for the Study of the Liver on the treatment of hepatitis B and C virus infections. Gastroenterol Hepatol 2006;29(Supl. 2):216-30. [ Links ]
14. European Association For The Study Of The L. EASL Clinical Practice Guidelines: management of chronic hepatitis B. J Hepatol 2009; 50:227-42. [ Links ]
15. Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology 2009;50:661-2. [ Links ]
16. Fontana RJ, Hann HW, Perrillo RP, Vierling JM, Wright T, Rakela J, et al. Determinants of early mortality in patients with decompensated chronic hepatitis B treated with antiviral therapy. Gastroenterology 2002;123:719-27. [ Links ]
17. Liaw YF. Management of patients with chronic hepatitis B. J Gastroenterol Hepatol 2002;17:406-8. [ Links ]
18. Liaw YF, Chien RN, Yeh CT, Tsai SL, Chu CM. Acute exacerbation and hepatitis B virus clearance after emergence of YMDD motif mutation during lamivudine therapy. Hepatology 1999;30:567-72. [ Links ]
19. Schiff E, Lai CL, Hadziyannis S, Neuhaus P, Terrault N, Colombo M, et al. Adefovir dipivoxil for wait-listed and post-liver transplantation patients with lamivudine-resistant hepatitis B: final long-term results. Liver Transpl 2007;13:349-60. [ Links ]
20. Marcellin P, Heathcote EJ, Buti M, Gane E, de Man RA, Krastev Z, et al. Tenofovir disoproxil fumarate versus adefovir dipivoxil for chronic hepatitis B. N Engl J Med 2008;359:2442-55. [ Links ]
21. Chan HL, Heathcote EJ, Marcellin P, Lai CL, Cho M, Moon YM, et al. Treatment of hepatitis B e antigen positive chronic hepatitis with telbivudine or adefovir: a randomized trial. Ann Intern Med 2007; 147:745-54. [ Links ]
22. Lange CM, Bojunga J, Hofmann WP, Wunder K, Mihm U, Zeuzem S, et al. Severe lactic acidosis during treatment of chronic hepatitis B with entecavir in patients with impaired liver function. Hepatology 2009;50:2001-6. [ Links ]
23. Shim JH, Lee HC, Kim KM, Lim YS, Chung YH, Lee YS, et al. Efficacy of entecavir in treatment-naive patients with hepatitis B virus-related decompensated cirrhosis. J Hepatol 2010;52:176-82. [ Links ]
24. Ratziu V, Thibault V, Benhamou Y, Poynard T. Successful rescue therapy with tenofovir in a patient with hepatic decompensation and adefovir resistant HBV mutant. Comp Hepatol 2006;5:1. [ Links ]
25. Jochum C, Gieseler RK, Gawlista I, Fiedler A, Manka P, Saner FH, et al. Hepatitis B-associated acute liver failure: immediate treatment with entecavir inhibits hepatitis B virus replication and potentially its sequelae. Digestion 2009;80:235-40. [ Links ]
26. Miyake Y, Iwasaki Y, Takaki A, Fujioka S, Takaguchi K, Ikeda H, et al. Lamivudine treatment improves the prognosis of fulminant hepatitis B. Intern Med 2008;47:1293-9. [ Links ]
27. Muller R, Gubernatis G, Farle M, Niehoff G, Klein H, Wittekind C, et al. Liver transplantation in HBs antigen (HBsAg) carriers. Prevention of hepatitis B virus (HBV) recurrence by passive immunization. J Hepatol 1991;13:90-6. [ Links ]
28. Samuel D, Bismuth A, Mathieu D, Arulnaden JL, Reynes M, Benhamou JP, et al. Passive immunoprophylaxis after liver transplantation in HBsAg-positive patients. Lancet 1991;337:813-5. [ Links ]
29. Chan HL, Chui AK, Lau WY, Chan FK, Hui AY, Rao AR, et al. Outcome of lamivudine resistant hepatitis B virus mutant post-liver transplantation on lamivudine monoprophylaxis. Clin Transplant 2004;18:295-300. [ Links ]
30. Mutimer D, Dusheiko G, Barrett C, Grellier L, Ahmed M, Anschuetz G, et al. Lamivudine without HBIg for prevention of graft reinfection by hepatitis B: long-term follow-up. Transplantation 2000;70:809-15. [ Links ]
31. Katz LH, Fraser A, Gafter-Gvili A, Leibovici L, Tur-Kaspa R. Lamivudine prevents reactivation of hepatitis B and reduces mortality in immunosuppressed patients: systematic review and meta-analysis. J Viral Hepat 2008;15:89-102. [ Links ]
32. Loomba R, Rowley AK, Wesley R, Smith KG, Liang TJ, Pucino F, et al. Hepatitis B immunoglobulin and lamivudine improve hepatitis B-related outcomes after liver transplantation: meta-analysis. Clin Gastroenterol Hepatol 2008;6:696-700. [ Links ]
33. Zheng S, Chen Y, Liang T, Lu A, Wang W, Shen Y, et al. Prevention of hepatitis B recurrence after liver transplantation using lamivudine or lamivudine combined with hepatitis B Immunoglobulin prophylaxis. Liver Transpl 2006;12:253-8. [ Links ]
34. Gane EJ, Angus PW, Strasser S, Crawford DH, Ring J, Jeffrey GP, et al. Lamivudine plus low-dose hepatitis B immunoglobulin to prevent recurrent hepatitis B following liver transplantation. Gastroenterology 2007;132:931-7. [ Links ]
35. Giusto M, Gentili F, Loria I, et al. Combined therapy with low dose hepatitis B immunoglobulin (HBIG) and lamivudine (LAM) in patients transplanted for HBV chronic liver disease: long terms efficacy and costs. J Hepatol 2008;48:S84. [ Links ]
36. Angus PW, Patterson SJ, Strasser SI, McCaughan GW, Gane E. A randomized study of adefovir dipivoxil in place of HBIG in combination with lamivudine as post-liver transplantation hepatitis B prophylaxis. Hepatology 2008;48:1460-6. [ Links ]
37. Caccamo L, Romeo R, Rossi G, Maggioni M, Radice F, Lunghi G, et al. No hepatitis recurrence using combination prophylaxis in HBV-positive liver transplant recipients with YMDD mutants. Transpl Int 2005;18:186-92. [ Links ]
38. Rosenau J, Bahr MJ, Tillmann HL, Trautwein C, Klempnauer J, Manns MP, et al. Lamivudine and low-dose hepatitis B immune globulin for prophylaxis of hepatitis B reinfection after liver transplantation possible role of mutations in the YMDD motif prior to transplantation as a risk factor for reinfection. J Hepatol 2001;34:895-902. [ Links ]
39. Marzano A, Lampertico P, Mazzaferro V, Carenzi S, Vigano M, Romito R, et al. Prophylaxis of hepatitis B virus recurrence after liver transplantation in carriers of lamivudine-resistant mutants. Liver Transpl 2005;11:532-8. [ Links ]
40. Buti M, Mas A, Prieto M, Casafont F, Gonzalez A, Miras M, et al. A randomized study comparing lamivudine monotherapy after a short course of hepatitis B immune globulin (HBIg) and lamivudine with long-term lamivudine plus HBIg in the prevention of hepatitis B virus recurrence after liver transplantation. J Hepatol 2003;38: 811-7. [ Links ]
41. Buti M, Mas A, Prieto M, Casafont F, Gonzalez A, Miras M, et al. Adherence to Lamivudine after an early withdrawal of hepatitis B immune globulin plays an important role in the long-term prevention of hepatitis B virus recurrence. Transplantation 2007;84:650-4. [ Links ]
42. Naoumov NV, Lopes AR, Burra P, Caccamo L, Iemmolo RM, de Man RA, et al. Randomized trial of lamivudine versus hepatitis B immunoglobulin for long-term prophylaxis of hepatitis B recurrence after liver transplantation. J Hepatol 2001;34:888-94. [ Links ]
43. Nath DS, Kalis A, Nelson S, Payne WD, Lake JR, Humar A. Hepatitis B prophylaxis post-liver transplant without maintenance hepatitis B immunoglobulin therapy. Clin Transplant 2006;20:206-10. [ Links ]
44. Wong SN, Chu CJ, Wai CT, Howell T, Moore C, Fontana RJ, et al. Low risk of hepatitis B virus recurrence after withdrawal of long-term hepatitis B immunoglobulin in patients receiving maintenance nucleos(t)ide analogue therapy. Liver Transpl 2007;13:374-81. [ Links ]
45. Yoshida H, Kato T, Levi DM, Regev A, Madariaga JR, Nishida S, et al. Lamivudine monoprophylaxis for liver transplant recipients with non-replicating hepatitis B virus infection. Clin Transplant 2007; 21:166-71. [ Links ]
46. Xi ZF, Xia Q, Zhang JJ, Chen XS, Han LZ, Wang X, et al. The role of entecavir in preventing hepatitis B recurrence after liver transplantation. J Dig Dis 2009;10:321-7. [ Links ]
47. Bienzle U, Gunther M, Neuhaus R, Vandepapeliere P, Vollmar J, Lun A, et al. Immunization with an adjuvant hepatitis B vaccine after liver transplantation for hepatitis B-related disease. Hepatology 2003;38:811-9. [ Links ]
48. Lo CM, Lau GK, Chan SC, Fan ST, Wong J. Efficacy of a pre-S containing vaccine in patients receiving lamivudine prophylaxis after liver transplantation for chronic hepatitis B. Am J Transplant 2007; 7:434-9. [ Links ]
49. Sanchez-Fueyo A, Rimola A, Grande L, Costa J, Mas A, Navasa M, et al. Hepatitis B immunoglobulin discontinuation followed by hepatitis B virus vaccination: A new strategy in the prophylaxis of hepatitis B virus recurrence after liver transplantation. Hepatology 2000;31:496-501. [ Links ]
50. Angelico M, Di Paolo D, Trinito MO, Petrolati A, Araco A, Zazza S et al. Failure of a reinforced triple course of hepatitis B vaccination in patients transplanted for HBV-related cirrhosis. Hepatology 2002; 35:176-81. [ Links ]
51. de Villa VH, Chen YS, Chen CL. Hepatitis B core antibody-positive grafts: recipient's risk. Transplantation 2003;75:S49-53. [ Links ]
52. Prieto M, Gomez MD, Berenguer M, Cordoba J, Rayon JM, Pastor M, et al. De novo hepatitis B after liver transplantation from hepatitis B core antibody-positive donors in an area with high prevalence of anti-HBc positivity in the donor population. Liver Transpl 2001; 7:51-8. [ Links ]
53. Yu AS, Vierling JM, Colquhoun SD, Arnaout WS, Chan CK, Khanafshar E, et al. Transmission of hepatitis B infection from hepatitis B core antibody-positive liver allografts is prevented by lamivudine therapy. Liver Transpl 2001;7:513-7. [ Links ]
54. Dickson RC, Everhart JE, Lake JR, Wei Y, Seaberg EC, Wiesner RH, et al. Transmission of hepatitis B by transplantation of livers from donors positive for antibody to hepatitis B core antigen. The National Institute of Diabetes and Digestive and Kidney Diseases Liver Transplantation Database. Gastroenterology 1997;113:1668-74. [ Links ]
55. Cholongitas E, Papatheodoridis GV, Burroughs AK. Liver grafts from anti-hepatitis B core positive donors: a systematic review. J Hepatol 2010;52:272-9. [ Links ]
56. Perrillo R. Hepatitis B virus prevention strategies for antibody to hepatitis B core antigen-positive liver donation: a survey of North American, European, and Asian-Pacific transplant programs. Liver Transpl 2009;15:223-32. [ Links ]
57. Prakoso E, Strasser SI, Koorey DJ, Verran D, McCaughan GW. Long-term lamivudine monotherapy prevents development of hepatitis B virus infection in hepatitis B surface-antigen negative liver transplant recipients from hepatitis B core-antibody-positive donors. Clin Transplant 2006;20:369-73. [ Links ]
58. Holt D, Thomas R, Van Thiel D, Brems JJ. Use of hepatitis B core antibody-positive donors in orthotopic liver transplantation. Arch Surg 2002;137:572-575; discussion 575-6. [ Links ]
59. Suehiro T, Shimada M, Kishikawa K, Shimura T, Soejima Y, Yoshizumi T, et al. Prevention of hepatitis B virus infection from hepatitis B core antibody-positive donor graft using hepatitis B immune globulin and lamivudine in living donor liver transplantation. Liver Int 2005;25:1169-74. [ Links ]
60. Roque-Afonso AM, Feray C, Samuel D, Simoneau D, Roche B, Emile JF, et al. Antibodies to hepatitis B surface antigen prevent viral reactivation in recipients of liver grafts from anti-HBC positive donors. Gut 2002;50:95-9. [ Links ]
61. Umeda M, Marusawa H, Ueda M, Takada Y, Egawa H, Uemoto S, et al. Beneficial effects of short-term lamivudine treatment for de novo hepatitis B virus reactivation after liver transplantation. Am J Transplant 2006;6:2680-5. [ Links ]
62. Takemura N, Sugawara Y, Tamura S, Makuuchi M. Liver transplantation using hepatitis B core antibody-positive grafts: review and university of Tokyo experience. Dig Dis Sci 2007;52:2472-7. [ Links ]
63. Alvarez-Suarez B, de-la-Revilla-Negro J, Ruiz-Antoran B, Calleja-Panero JL. Hepatitis B reactivation and current clinical impact. Rev Esp Enferm Dig 2010;102:542-52. [ Links ]
64. Davies SE, Portmann BC, O'Grady JG, Aldis PM, Chaggar K, Alexander GJ, et al. Hepatic histological findings after transplantation for chronic hepatitis B virus infection, including a unique pattern of fibrosing cholestatic hepatitis. Hepatology 1991;13:150-7. [ Links ]
65. Thung SN. Histologic findings in recurrent HBV. Liver Transpl 2006;12:S50-53. [ Links ]
66. Terrault N, Roche B, Samuel D. Management of the hepatitis B virus in the liver transplantation setting: a European and an American perspective. Liver Transpl 2005;11:716-32. [ Links ]
67. Natsuizaka M, Hige S, Ono Y, Ogawa K, Nakanishi M, Chuma M, et al. Long-term follow-up of chronic hepatitis B after the emergence of mutations in the hepatitis B virus polymerase region. J Viral Hepat 2005;12:154-9. [ Links ]
68. Akyildiz M, Karasu Z, Zeytunlu M, Aydin U, Ozacar T, Kilic M. Adefovir dipivoxil therapy in liver transplant recipients for recurrence of hepatitis B virus infection despite lamivudine plus hepatitis B immunoglobulin prophylaxis. J Gastroenterol Hepatol 2007; 22: 2130-4. [ Links ]
69. Limquiaco JL, Wong J, Wong VW, Wong GL, Tse CH, Chan HY, et al. Lamivudine monoprophylaxis and adefovir salvage for liver transplantation in chronic hepatitis B: a seven-year follow-up study. J Med Virol 2009;81:224-9. [ Links ]
70. Lilly L, Therapondos G, Mason AL, Kelly W, Burak W. Tenofovir is effective in suppressing HBV replication in liver transplant recipients with nucleos(t)ide resistant recurrent disease. Hepatology 2008; 48:574A. [ Links ]
71. Neff GW, Nery J, Lau DT, O'Brien CB, Duncan R, Shire NJ, et al. Tenofovir therapy for lamivudine resistance following liver transplantation. Ann Pharmacother 2004;38:1999-2004. [ Links ]
72. Sherman M, Yurdaydin C, Sollano J, Silva M, Liaw YF, Cianciara J, et al. Entecavir for treatment of lamivudine-refractory, HBeAg-positive chronic hepatitis B. Gastroenterology 2006;130:2039-49. [ Links ]
![]() Correspondence:
Correspondence:
J. Ignacio Herrero.
Unidad de Hepatología.
Clínica Universidad de Navarra.
Av Pío XII 36.
34008 Pamplona. Navarra, Spain
e-mail: iherrero@unav.es
Received: 20-05-10.
Accepted: 23-12-10.











 texto em
texto em