Meu SciELO
Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Revista Española de Enfermedades Digestivas
versão impressa ISSN 1130-0108
Rev. esp. enferm. dig. vol.109 no.5 Madrid Mai. 2017
https://dx.doi.org/10.17235/reed.2017.4466/2016
CASE REPORT
Cutaneous necrosis secondary to terlipressin therapy. A rare but serious side effect. Case report and literature review
Necrosis cutánea secundaria a terlipresina, un raro pero grave efecto secundario. Reporte de un caso y revisión de la literatura
Enrique Iglesias-Julián1, Ester Badía-Aranda2, Belén Bernad-Cabredo2, Daniel Corrales-Cruz3 and María J. Romero-Arauzo2
Departments of 1Internal Medicine, 2Digestive Diseases and 3Pathology. Hospital Universitario de Burgos. Burgos, Spain
ABSTRACT
Terlipressin is a vasopressin analogue used in esophageal variceal bleeding and hepatorenal syndrome management. It is a safe drug with mild secondary effects. However, potentially serious ischemic complications may occur, such as cutaneous necrosis. It is useful to recognize these events early, in order to withdraw terlipressin and introduce other adjuvant drugs if needed. We report a detailed case of cutaneous necrosis secondary to terlipressin administration and present a case review of patients, describing their characteristics, risk factors, lesion locations, doses, methods of administration and possible treatments.
Key words: Terlipressin. Cutaneous necrosis. Side effect.
RESUMEN
La terlipresina es un análogo de la vasopresina, utilizado tanto en el tratamiento de la hemorragia por varices esofágicas como en el síndrome hepatorrenal. En general es un fármaco seguro, con efectos secundarios leves. Sin embargo, en ocasiones pueden ocurrir complicaciones isquémicas potencialmente graves, como la necrosis cutánea, que es necesario reconocer de forma precoz para realizar la retirada inmediata del fármaco. Presentamos el caso de una paciente que presentó necrosis cutánea extensa secundaria a la administración de terlipresina, y realizamos una revisión de los casos publicados, describiendo sus características, posibles factores de riesgo, localización de las lesiones, dosis recibida, forma de administración y posibles tratamientos.
Palabras clave: Terlipresina. Necrosis cutánea. Efecto secundario.
Introduction
Terlipressin is a vasopressin analogue used in the management of esophageal variceal bleeding (OVB) and hepatorenal syndrome (HRS). Undesirable effects are usually mild. However, secondary ischemic events such as cutaneous necrosis may occur, which can be potentially dangerous.
Case report
An 84-year-old woman with a medical history of alcoholic and hepatitis C virus (HCV) cirrhosis and large esophageal varices due to portal hypertension (in treatment with propranolol as primary prophylaxis of bleeding) was admitted because of OVB. In addition, she had arterial hypertension, chronic renal failure and auricular fibrillation, for which she was anticoagulated with acenocumarol. OVB was resolved after endoscopic ligation and treatment with intravenous bolus of terlipressin was initiated (1.5 mg/4 h).
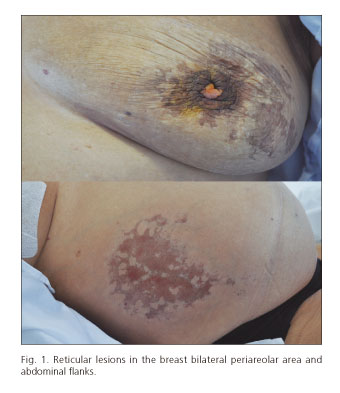
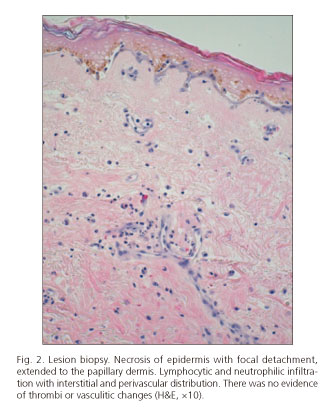
Twenty-four hours after the start of vasoactive therapy, the patient suffered from diffuse abdominal pain. At that point, spontaneous bacterial peritonitis and intestinal ischemia were excluded. One day later, hematoma and bullous lesions were seen over the hypogastrium, and blackish-brown, reticular shaped lesions were observed in the flanks and periareolar regions (Fig. 1) during the following few days. Cutaneous biopsy revealed necrosis of the epidermis and focal detachment that extended into the papillary dermis. Lymphocytic and neutrophilic infiltrate with interstitial and perivascular distribution was seen in the superficial dermis without evidence of thrombi or vasculitic signs. Hematic interstitial extravasation was observed and immunofluorescence was negative (Fig. 2).
Terlipressin therapy was suspended due to the suspicion of cutaneous necrosis secondary to its administration. Cutaneous lesions evolved until dermal detachment and the appearance of granulation tissue, with early improvement within a few weeks. Both the patient and her daughter consented to the publication of this case and photographs.
Discussion
Terlipressin is an arginin-vasopressin analogue with high V1-receptor affinity, which are mainly found in the smooth muscle within the splanchnic circulation vessels and also in the systemic circulation, kidneys and heart. Its undesirable effects are usually mild (paleness, hypertension, bradycardia, headache, abdominal pain, diarrhea and hyponatremia), and patient's rarely present ischemic events such as intestinal ischemia, muscular and myocardial infarction or skin necrosis.
The appearance of skin lesions has been reported in 21 cases within the literature (2-9), including the one currently presented. Terlipressin indication was HRS in 62% and OVB in 38% of cases, mean age was 62.3 ± 12.1 (39-84) years and 76% were men. Cirrhosis etiology was: alcoholic in 52%, HCV in 19%, non-alcoholic fatty liver disease in 14%, hepatitis B infection in one case and autoimmune liver disease in another.
Some possible risk factors have been proposed, but obesity was the most prevalent within these cases, present in 29% of cases (it could have been present in more cases but may not have always been reported). Regarding arteriopathy, cardiac ischemia was present in 14% and previous stroke in one case, but no cases with peripheral arteriopathy were reported. Other proposed risk factors, such as venous insufficiency, diabetes or spontaneous bacterial peritonitis were found in one case each, so we cannot confirm them as predisposing factors.
The mean time from terlipressin administration to the appearance of skin lesions was 2.74 ± 2.28 (0.5-11) days. While lesions appeared during treatment in 20 of the reported cases, one case reported the appearance of lesions two days following terlipressin withdrawal.
Terlipressin was administrated in bolus in 90.5% of cases, in varying doses of 0.5, 1 or 1.5 mg every four to six hours. The average dose was 5.21 ± 2.90 (2-12) mg/day. In two cases, continuous perfusion was used with 9 and 12 mg/day respectively. A recently published study (10) suggests that continuous infusion could avoid secondary effects, as lower doses of the drug are needed. However, none of the patients within this study presented with cutaneous necrosis; they only report one isolated peripheral ischemia case within the continuous infusion group. Nevertheless, in the patients we analyzed, the ones treated with bolus received lower total daily doses of terlipressin than the patients on continuous infusion. The total dose from which the skin lesions appeared was highly variable (14.34 ± 15.9 [4-66] mg) so we cannot relate this effect to the drug accumulation. Based on that, it is difficult to comment on the association with total daily dose or route of administration.
Skin lesions involved the thighs in 62% of cases, abdomen in 48% (generally in flanks) and scrotum in 38% of men. Other less affected areas were toes, breasts, back, forearms (excluding hand and fingers), scalp and tongue. Unlike other vasoactive drugs where ischemic changes occur at distal areas with progression to proximal areas, only 18% of the cases reported with terlipressin had acral involvement. Arteriopathy should be an expected risk factor for the appearance of secondary adverse events with terlipressin due to its vasoconstrictor effect. However, it was not a common observation in our case studies. As obesity is frequent within these patients, with a reported predominance of thighs and abdomen lesions, some authors have postulated that this could be explained by a greater amount of V1 receptors in the adipose tissue. This assumption is consistent with the breast damage observed (two in five women) but does not explain the common scrotum injury in men, scalp or other areas with less adipose tissue. At this point, the atypical location of the lesions is probably based on the heterogeneous distribution of V1 receptors within the body, but this uncertainty still remains.
The reported histopathological studies were unspecific, similar to the one presented in our case (Fig. 2), but they are useful to rule out other pathologies, mainly vasculitis. These lesions present a characteristic blackish geographical shape (Fig. 1), which should raise suspicion in patients being treated with terlipressin.
When cutaneous necrosis is suspected, terlipressin treatment must be suspended. In this context, 65% of the cases improved after terlipressin withdrawal. When unfavorable evolution occurred, treatment with alprostadile (10 µg/day) (6) and sildenafil 50 mg/12 h (6,9) has been used. Nitrates have also been proposed in this context (4).
It is important to point out that, amongst the reported cases, mortality within 30 days was 71%, mostly not related to cutaneous necrosis but secondary to complications related to the advanced liver disease of patients receiving terlipressin.
Conclusion
Secondary cutaneous necrosis due to terlipressin is a rare but serious side-effect. It is more frequent in obese patients. It does not seem to be dependent on the administration method (perfusion or bolus) nor on daily or accumulative dose. Lesions have characteristic locations that usually respect distal areas. Early identification is essential for the immediate withdrawal of the drug, which will result in improvement of the lesions in a large percentage of cases, whilst others may require the use of complementary treatments.
References
1. Holmes CL, Landry DW, Granton JT. Science review: Vasopressin and the cardiovascular system part 1 - Receptor physiology. Crit Care 2003;7:427-34. DOI: 10.1186/cc2337. [ Links ]
2. Di Micoli A, Bracci E, Cappa FM, et al. Terlipressin infusion induces ischemia of breast skin in a cirrothic patient with hepatorenal syndrome. Dig Liver Dis 2008;40:304-5. DOI: 10.1016/j.dld.2007.11.009. [ Links ]
3. Posada C, Feal C, García-Cruz A, et al. Cutaneous necrosis secondary to terlipressin therapy. Acta Derm Venereol 2009;89:434-5. DOI: 10.2340/00015555-0651. [ Links ]
4. Yefet E, Gershovich M, Farber E, et al. Extensive epidermal necrosis due to terlipressin. Isr Med Assoc J 2011;13:180-1. [ Links ]
5. Lu YY, Wei KC, Wu CS. Terlipressin-induced extensive skin necrosis: A case report and published work review. J Dermatol 2012;39:866-8. DOI: 10.1111/j.1346-8138.2012.01595.x. [ Links ]
6. Lee HJ, Oh MJ. A case of peripheral gangrene and osteomyelitis secondary to terlipressin therapy in advanced liver disease. Clin Mol Hepatol 2013;19:179-84. DOI: 10.3350/cmh.2013.19.2.179. [ Links ]
7. Taşliyurt T, Kutlutürk F, Erdemir F, et al. Ischemıc skin necrosis following terlipressin therapy: Report of two cases and review of the literature. Turk J Gastroenterol 2012;23(6):788-91. DOI: 10.4318/tjg.2012.0490. [ Links ]
8. Herrera J, Leiva-Salinas M, Palazón JM, et al. Extensive cutaneous necrosis due to terlipressin use. Gastroenterol Hepatol 2015;38(1):12-3. DOI: 10.1016/j.gastrohep.2014.03.014. [ Links ]
9. Bañuelos Ramírez DD, Sánchez Alonso S, Ramírez Palma MM. Sildenafil in severe peripheral ischemia induced by terlipressin. A case report. Reumatol Clin 2011;7(1):59-60. DOI: 10.1016/j.reuma. 2010.05.005. [ Links ]
10. Cavallin M, Piano S, Romaro A, et al. Terlipressin given by continuous intravenous infusion versus intravenous boluses in the treatment of hepatorenal syndrome: A randomized controlled study. Hepatol 2016;63(3):983-92. DOI: 10.1002/hep.28396. [ Links ]
![]() Correspondence:
Correspondence:
Enrique Iglesias Julián.
Department of Internal Medicine.
Hospital Universitario de Burgos.
Avda. Islas Baleares, 3.
09006 Burgos, Spain
e-mail: eiglesiasjulian@gmail.com
Received: 27-05-2016
Accepted: 01-06-2016











 texto em
texto em