Meu SciELO
Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Revista Española de Enfermedades Digestivas
versão impressa ISSN 1130-0108
Rev. esp. enferm. dig. vol.110 no.4 Madrid Abr. 2018
https://dx.doi.org/10.17235/reed.2018.5262/2017
ORIGINAL PAPERS
The safety of deep sedation with propofol controlled by the endoscopist in endoscopic retrograde cholangiopancreatography (ERCP): a prospective study in a tertiary hospital
1Servicio de Aparato Digestivo. Hospital Universitario Miguel Servet. Zaragoza. Spain
2Servicio de Medicina Preventiva y Salud Pública. Hospital Universitario Miguel Servet. Zaragoza. Spain
INTRODUCTION
Sedation in digestive endoscopy is an indicator of quality, as it improves tolerance to the procedure and allows the endoscopist to perform the exploration under better conditions and to achieve the objective. Sedation levels range from light sedation or anxiolysis to general anesthesia, and should be adjusted to each patient and procedure to ensure safety, comfort and technical success 1,2. Propofol has become the drug of choice in the last decade, especially in complex and prolonged techniques such as ERCP, due to its short-acting hypnotic action, short half-life and rapid post-sedation recovery. The most frequent secondary events are respiratory depression, hypotension, and bradycardia. This fact and the absence of an antidote mean that this drug is also considered on occasions as exclusively used by anesthetists or intensivists. Several recently published articles support the safe and effective administration of propofol during digestive endoscopy by a nurse, whilst under the supervision of the endoscopist, provided that both have an adequate level of training 1,2,3,4,5,6,7,8,9,10. However, the availability of literature on the use of propofol in advanced endoscopy and the factors related to the development of adverse events is limited. Therefore, this study aimed to assess the safety of sedation with propofol controlled by the endoscopist in ERCP, a procedure of great technical complexity and, in most cases, performed in patients of advanced age with a high anesthetic risk.
This study aimed to assess the safety of deep sedation with endoscopist-controlled propofol in patients undergoing endoscopic retrograde cholangiopancreatography (ERCP) and to identify factors associated with the occurrence of adverse events due to sedation (AES).
MATERIAL AND METHODS
Type of study and sample
This is a prospective study of patients undergoing ERCP under deep sedation with propofol controlled by the endoscopist, from June 2014 to June 2016, in the Digestive Endoscopy Unit of the Hospital Universitario Miguel Servet of Zaragoza.
Data collection
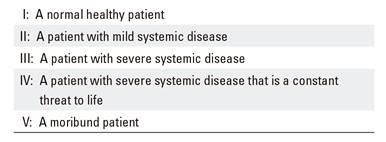
A data collection sheet was created before starting the procedure that included the following variables: affiliation data, age, weight, height, body mass index (BMI), date of the examination, allergies (medicines and food), origin (emergency or inpatient), priority (urgent or scheduled), reason for the exploration, Mallampati score, pre-anesthetic evaluation of the physical state according to the American Society of Anesthesiology (ASA) (Table 1), induction dose and total propofol dose and the duration of the exploration and adverse events.
Table 1. Pre-anesthetic evaluation of the physical state according to the American Society of Anesthesiology (ASA)
Concomitant diseases were classified as follows: respiratory diseases (asthma, chronic obstructive pulmonary disease, obstructive sleep apnea syndrome), cardiovascular (ischemic heart disease, moderate-severe valvulopathy, arrhythmia, arterial hypertension), diabetes mellitus and renal failure.
Sedation procedure
The characteristics of the intervention and sedation and the possible associated risks were explained to all patients and/or relatives. All participants signed a specific informed consent.
The explorations were performed by an endoscopist and two nurses, all of whom were trained in sedation with propofol and basic and advanced cardiopulmonary resuscitation. One of the nurses was exclusively responsible for the management and control of sedation, according to the instructions of the endoscopist. The constants were recorded at the beginning and end of the procedure and blood pressure (HR), heart rate (HR) and oxygen saturation (SatO2) were continuously monitored during the procedure. A nasal cannula was set to deliver 2-3 l/min of oxygen in all cases. Fifty-milliliter vials containing 500 mg of propofol (10 mg/ml) were used. Propofol was administered by induction bolus (0.5-1 mg/kg) and subsequently by continuous infusion pump, with variable doses depending on the patients' age, weight and clinical conditions.
A sedation adverse event (AES) was defined as follows:
Hypotension: systolic blood pressure (SBP) less than 90 mmHg or a decrease of > 25 mmHg compared with the initial SBP.
Bradycardia: a decrease in HR below 50 beats per minute.
Hypoxemia: a fall in O2 saturation below 90%.
The additional maneuvers that were necessary for the resolution of the AES were recorded, including infusion of physiological saline (PS) or administration of inotropes, administration of atropine, increased O2 flow, head tilt-chin lift maneuver, ventimask, bag valve mask or orotracheal intubation.
Cases in which the exploration was interrupted as a consequence of more serious complications, such as laryngospasm, airway obstruction, bronchoaspiration or tachycardia, were also recorded.
Statistical analysis
A descriptive analysis was performed to evaluate the frequency of ESA in the patient cohort. All numerical variables were expressed as median values (p25-p75) and categorical variables were expressed as absolute and relative frequencies as a percentage. Patients were classified into two groups: under 65 and over 65 years of age. A univariate analysis of clinical predictors, including age, sex, BMI, ASA, exploration time, propofol dose at induction (mg) and total administered dose (mg) was performed. The association between quantitative variables was evaluated using the Wilcoxon-Mann-Whitney test for non-parametric variables. Categorical variables were assessed using the Chi-squared test or Fisher's exact test. Statistical significance was set at p < 0.05. A multivariate logistic regression analysis was performed to evaluate independent predictors of SAE, which included variables in which a significant association was found by univariate analysis. The analyses were performed using the SPSS 20.0 statistical package.
RESULTS
A total of 661 patients underwent ERCP with propofol sedation over a 24-month period. An initial analysis of the patient cohort is summarized in table 2 which includes patient characteristics, the procedure and the anesthetic used (propofol). The median age of the cohort was 78.8 years (interquartile range 66.7-85.2); 48% were male and 52% were female. The median BMI was 25.6 (interquartile range 23.1-28.3). With regard to the ASA risk, 80 patients (12.1%) were ASA I, 373 patients (56.4%) were ASA II, 192 patients (29%) were ASA III, and 16 patients (2.4%) were ASA IV. The median duration of the exploration was 30 minutes (interquartile range 20-45). The median propofol induction dose was 60 mg (interquartile range 50-80) and the total dose was 200 mg (interquartile range 140-300).
Table 2 Characteristics of the patient and examination
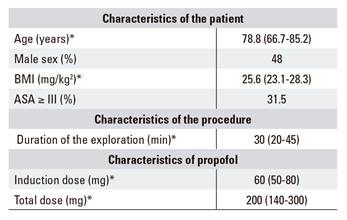
*Numerical variables are expressed in median-interquartile range. Categorical variables are expressed as a percentage.
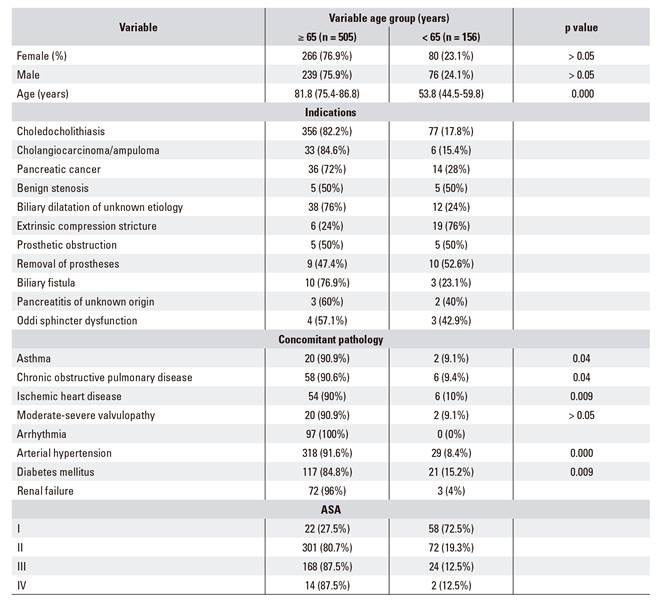
The patient cohort was divided into two groups, those younger than 65 years and those 65 years or older, in order to evaluate possible differences between younger and older patients. The median age of patients in the younger than 65 group was 53.8 years of age (44.5-59.8), and the median age in the ≥ 65 group was 81.8 years of age (75.4-86.8). The most common identified pathology was choledocholithiasis, which was more common in older patients (82.2% vs 17.8%), followed by tumor pathology. A similar trend was observed with regard to comorbidity, with more concomitant pathology in the ≥ 65 years group, which was statistically significant (p < 0.05) (Table 3).
Hypoxemia, hypotension and bradycardia were evaluated as AES in the global patient cohort, with a total of 64 patients (9.7%) (Table 4). Hypoxemia was the most frequent adverse event in 38 patients (5.7%), followed by bradycardia in 16 patients (2.4%) and hypotension in ten cases (1.6%). With regard to hypoxemia as the most frequent AES, an additional maneuver was required in all cases for recovery, this included increased flow in six patients (0.9%), a head tilt-chin lift maneuver in 21 patients (3.2%) and a mask in 11 cases (1.7%). More aggressive measures were not required, such as bag valve mask or orotracheal intubation (OTI). Bradycardia was recorded in 16 patients (2.4%), of whom six (0.9%) recovered spontaneously and ten (1.5%) required atropine. Of the ten patients with arterial hypotension (1.6%), nine (1.4%) responded to fluid therapy and one (0.2%) required an infusion of inotropes. Of the 661 patients in the global sample cohort, only 16 patients (2.4%) had tachycardia and there was one death of an elderly patient with recurrent cholangitis due to choledocholithiasis, severe cardiac disease and ASA IV. This death occurred as a result of ventricular fibrillation (VF) during a complex ERCP, which required previous duodenal dilatation.
Table 4 Adverse events to sedation
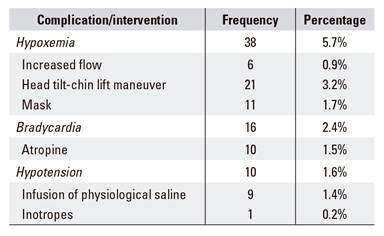
*The presence of a single adverse event due to sedation was considered as an anesthetic complication. The registered interventions were not counted more than once if the same maneuver was repeated in the same patient.
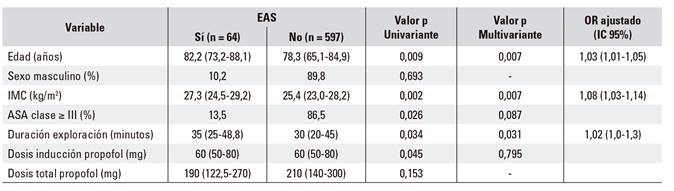
Clinical, endoscopic and pharmacological characteristics were evaluated in order to determine the predictive factors of ASD (Table 5). According to the univariate analysis, patients who developed AES were more likely to be ASA ≥ III (p = 0.026), older (p = 0.009), have a higher BMI (p = 0.002) and a longer exploration time (p = 0.034). The induction dose of propofol was associated with a greater likelihood of adverse events (p = 0.045) but not the total dose of propofol administered (p = 0.153). A multivariate logistic regression analysis based on the univariate analysis was performed in order to evaluate the independent predictors of BP. Age (odds ratio [OR]: 1.03 [95% CI: 1.01-1.05], p = 0.007), BMI (OR: 1.08 [95% CI: 1.03-1.14], p = 0.007) and the duration of the exploration (OR: 1.02 [95% CI: 1.3], p = 0.031) were identified as independent predictors of AS. However, the adjusted ORs (95% CI) were low.
DISCUSSION
Sedation in digestive endoscopy is traditionally performed with benzodiazepines, either with or without opiates 11,12,13. Propofol (2-6 diisopropylphenol) is an ultrashort-acting sedative and hypnotic drug 1,3,4,7,13 that offers multiple advantages compared with traditional sedation in endoscopic procedures due to its short half-life and rapid post-sedation recovery 3,4,7,9,14. The absence of an antidote and the possibility of the development of cardiorespiratory complications has made the use of propofol by non-anesthesiologists controversial. However, not all centers (including our center) routinely have an anesthetist available to perform the sedation during the endoscopy. In addition, there is extensive literature supporting the safety of sedation with propofol in digestive endoscopy performed by non-anesthetist medical personnel that are trained in the use of the drug 1,11,12,15,16,17.
The characteristics of the exploration and the risk to the patient should be taken into consideration. Low complexity diagnostic examinations can be performed in non-risk ASA I and II patients without the need for additional sedation staff. In complex therapeutic procedures and/or those involving ASA III-IV patients or patients with a sedation risk (short neck, sleep apnea, severe decompensated chronic diseases, among other conditions), adverse events due to sedation are more frequent. In these cases, it is highly recommended to have a qualified person dedicated exclusively to sedation 1,6.
The ESGE Clinical Practice Guide recommends the participation of an anesthesiologist for ASA class ≥ III patients and in cases where a prolonged procedure is anticipated. However, the article published in 2016 by Pérez Cuadrado et al. concluded that sedation performed by endoscopy staff for complex and potentially lengthy explorations such as ERCP was feasible and safe in experienced units, even in patients with ASA III and IV 18.
The management of sedation in digestive endoscopy differs widely from country to country, from one health system to another and also depends on the local circumstances and the preferences of the patient and the endoscopist. Optimal sedation should be individually tailored to the patient and should be based on the balance between clinical risk and the type of procedure performed 7,11,12,13.
ERCP is considered one of the most complex procedures of digestive endoscopy (4,6). Patients of an advanced age, a prone or semi-prone position and a long duration of the procedure make sedation more difficult and the occurrence of adverse events more frequent. The literature on propofol sedation in advanced endoscopy techniques such as ERCP is scarce. Meta-analyses by Bo LL et al. and Sethi et al. analyzed the safety and efficacy of propofol sedation in ERCP. They demonstrated that sedation induction is faster with propofol, the quality of sedation and patient collaboration is better and the recovery time is significantly reduced compared with traditional sedation, without increasing the incidence of hypotension or hypoxia 4,19.
The rate of adverse events in this study was in accordance with the data reported in the literature. Coté et al. conducted a study of 799 patients undergoing advanced endoscopy over a seven-month period. Of these, 423 patients underwent echo-endoscopy (52.9%), 336 ERCP (42.1%) and 40 enteroscopy. Male gender, mass index (BMI) and ASA III class or higher were independent predictors of the need for maneuvers on the respiratory tract. The hypoxemia rate was 12.8% versus 5.7% in our case and the hypotension rate was 0.5% compared with 1.6%. However, it is important to note that not all patients underwent an ERCP and that 61% were ASA III or higher, compared with 31.5% in our study. They also did not require invasive rescue maneuvers with OTI. The exploration times in both cases were similar, 30 ± 19 vs 30 (20-45) minutes 8,11.
The study by Yang JF. et al. with a cohort of 3,040 patients is the largest analysis performed to date that evaluated the safety of propofol in ERCP. In this study, sedation was performed by a trained nurse under the supervision of an anesthesiologist. Sedation was performed with propofol alone or in combination with low doses of opioid or benzodiazepines. Airway manipulation due to hypoxemia occurred in 28% of patients and 1.6% required OTI. Almost half of the patients were ASA III or higher, and the endoscopy time was longer than that observed in our study, with a mean time of 59 ± 23 min. Multivariate regression analysis showed that males, ASA III or higher, advanced age, increased BMI and duration of the exploration were independent predictors of AES. The association of the latter three factors with AES was also observed in our study 9.
ERCP is frequently performed in the elderly and those with comorbidities. However, evaluations of the use of propofol in elderly and high-risk patients undergoing ERCP are scarce. The study by Riphaus et al. included 150 consecutive patients older than 80 years with a high comorbidity (ASA ≥ III: 91%) who randomly received midazolam plus meperidine (n = 75) or propofol alone (n = 75). A lower number of adverse events were detected in relation to propofol desaturation, 12% vs 26% with midazolam and meperidine (p < 0.001). No differences were found with regard to the frequency of hypotension 5,10,13, 9% with midazolam/meperidine vs 11% with propofol. The frequency of hypotension with propofol was more frequent than that observed in our study (11% vs 1.6%). However, a limit of < 100 mmHg was set to define hypotension. The studies by de Rex et al. and Walker et al. report similar data 10.
Nonaka M et al. published a retrospective study in Japan of elderly patients undergoing therapeutic endoscopy. The results support the safe use of propofol by the gastroenterologist and the rate of adverse events was similar to that seen with young patients. The patients selected were ASA I and II and those who had respiratory comorbidity were excluded. Therefore, their results should be interpreted with caution and could not be extrapolated to our sample, which included ASA III and IV patients 14.
In an analysis of risk factors, Wehrmann et al. identified a total dose of propofol > 100 mg as an independent risk factor for secondary events related to sedation. This could be attributed to a longer procedure duration, although this could not be verified from the study as the duration of the exploration was not systematically recorded. In contrast to the Wehrmann study, the duration of exploration in our study was considered as an independent risk factor (p = 0.031) but not the total dose of propofol 6,20.
In conclusion, the results of this study indicate that the use of propofol administered by trained endoscopy personnel is safe in advanced endoscopic procedures, such as ERCP. This is also the case for patients at high anesthetic risk, such as the elderly and those with ASA III-IV. The number of AES was low and most were resolved spontaneously or were treated with simple and non-invasive maneuvers. Age, ASA, BMI, duration of exploration and the induction dose of propofol were associated with an increased likelihood of SAE. Future studies are likely to identify new additional clinical predictors that will help clinicians to better select patients for this procedure. However, we believe that multidisciplinary collaboration between gastroenterologists and anesthesiologists is necessary and that the sedation protocol should be elaborated to offer the best available option that is adapted to the individual characteristics of each patient.
BIBLIOGRAFÍA
1. Igea F, Casellas JA, González-Huix F, et al. Sedación en endoscopia digestiva. Guía de práctica clínica de la Sociedad Española de Endoscopia Digestiva. Rev Esp Enferm Dig 2014;106:195-211. [ Links ]
2. Garewal D, Waikar P. Propofol sedation for ERCP procedures: a dilemma? Observations from an anesthesia perspective. Diagn Ther Endosc 2012; 2012:639190. [ Links ]
3. Paulo S. Sedation in gastrointestinal endoscopy: a prospective study comparing nonanesthesiologist-administered propofol and monitored anesthesia care. Endosc Int Open 2015:7-13. [ Links ]
4. Bo LL, Bai Y, Bian J-J, et al. Propofol vs traditional sedative agents for endoscopic retrograde cholangiopancreatography: a meta-analysis. World J Gastroenterol 2011;17(30):3538-43. [ Links ]
5. Cheriyan DG, Byrne MF. Propofol use in endoscopic retrograde cholangiopancreatography and endoscopic ultrasound. World J Gastroenterol 2014;20(18):5171-6. [ Links ]
6. Chainaki IG, Manolaraki MM, Paspatis GA. Deep sedation for endoscopic retrograde cholangiopacreatography. World J Gastrointest Endosc 2011;3(2):34-9. [ Links ]
7. Burtea DE, Dimitriu A, Malos AE, et al. Current role of non-anesthesiologist administered propofol sedation in advanced interventional endoscopy. World J Gastrointest Endosc 2015;7(10):981-6. [ Links ]
8. Coté GA, Hovis RM, Ansstas MA, et al. Incidence of sedation-related complications with propofol use during advanced endoscopic procedures. Clin Gastroenterol Hepatol 2010;8(2):137-42. [ Links ]
9. Yang JF, Farooq P, Zwilling K, et al. Efficacy and safety of propofol-mediated sedation for outpatient endoscopic retrograde cholangiopancreatography (ERCP). Dig Dis Sci 2016;61(6):1686-91. [ Links ]
10. Riphaus A, Stergiou N, Wehrmann T. Sedation with propofol for routine ERCP in high-risk octogenarians: a randomized, controlled study. Am J Gastroenterol 2005;100(9):1957-63. [ Links ]
11. Riphaus A, Beilenhoff U, Vilmann P, et al. European curriculum for sedation training in gastrointestinal endoscopy: position statement of the European Society of Gastrointestinal Endoscopy (ESGE) and European Society of Gastroenterology and Endoscopy Nurses and Associates (ESGENA). Endoscopy 2013;45(6)496-504. [ Links ]
12. Dumonceau JM, Riphaus A, Aparicio JR, et al. Non-anesthesiologist administration of propofol for gastrointestinal endoscopy: European Society of Gastrointestinal Endoscopy, European Society of Gastroenterology and Endoscopy Nurses and Associates Guideline - Updated June. Endoscopy 2015;47:1175-89. DOI: 10.1055/s-0034-1393414 [ Links ]
13. Triantafillidis JK, Merikas E, Nikolakis D, et al. Sedation in gastrointestinal endoscopy: current issues. World J Gastroenterol 2013;19(4):463-81. [ Links ]
14. Nonaka M, Gotoda T, Kusano C, et al. Safety of gastroenterologist-guided sedation with propofol for upper gastrointestinal therapeutic endoscopy in elderly patients Compared with younger patients. Gut Liver 2015;9(1):38-42. DOI: 10.5009/gnl13368 [ Links ]
15. Qadeer MA, Vargo JJ, Khandwala F, et al. Propofol versus traditional sedative agents for gastrointestinal endoscopy: a meta-analisis. Clin Gastroenterol Hepatol 2005;3(11):1049-56. DOI: 10.1016/S1542-3565(05)00742-1 [ Links ]
16. Wang D, Chen C, Chen J, et al. The use of propofol as a sedative agent in gastrointestinal endoscopy: a meta-analysis. PLoS One 2013;8(1):e53311. DOI: 10.1371/journal.pone.0053311 [ Links ]
17. Lichtenstein DR, Jagannath S, Baron TH, et al. Sedation and anesthesia in GI endoscopy. Gastrointest Endosc 2008;68(5):815-26. [ Links ]
18. Pérez-Cuadrado-Robles E, González-Ramírez A, Lancho-Seco Á, et al. Safety and risk factors for difficult endoscopist-directed ERCP sedation in daily practice: a hospital-based case-control study. Rev Esp Enferm Dig 2016;108:240-5. [ Links ]
19. Sethi S, Wadhwa V, Thaker A, et al. Propofol versus traditional sedative agents for advanced endoscopic procedures: a meta-analysis. Dig Endosc 2014;26(4):515-24. DOI: 10.1111/den.12219 [ Links ]
20. Wehrmann T, Riphaus A. Sedation with propofol for interventional endoscopic procedures: a risk factor analysis. Scand J Gastroenterol 2009; 43(3):368-74. [ Links ]
Received: September 21, 2017; Accepted: January 02, 2018











 texto em
texto em