Meu SciELO
Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Revista Española de Enfermedades Digestivas
versão impressa ISSN 1130-0108
Rev. esp. enferm. dig. vol.110 no.4 Madrid Abr. 2018
https://dx.doi.org/10.17235/reed.2018.5283/2017
ORIGINAL PAPERS
Quality of sedation with propofol administered by non-anesthetists in a digestive endoscopy unit: the results of a one year experience
1Unidad de Pruebas Funcionales Digestivas, Agencia Sanitaria de la Costa del Sol. Marbella, Málaga, España
2Departamento de Aparato Digestivo. Agencia Sanitaria de la Costa del Sol. Marbella, Málaga, España
3Dirección de Enfermería, Agencia Sanitaria de la Costa del Sol. Marbella, Málaga, España
4Área de Reanimación y Anestesia. Agencia Sanitaria de la Costa del Sol. Marbella, Málaga, España
5Unidad de Enfermería de Digestivo. Agencia Sanitaria de la Costa del Sol. Marbella, Málaga, España
INTRODUCTION
In the last 20 years, numerous studies have established that non-anesthetist physicians and nurses can apply propofol sedation safely and effectively to patients undergoing endoscopic procedures 1,2,3,4. According to the guidelines of the Spanish Society of Digestive Endoscopy (SEED) 5, a properly trained team can perform propofol sedation in non-complex, non-invasive diagnostic examinations for patients classed as ASA I-II, without risk and without requiring the presence of personnel that exclusively provide sedation. Thus, the anesthetist can be freed to address complex therapeutic procedures and/or those performed in patients with higher levels of ASA risk. ASA I corresponds to a healthy patient with no physical or metabolic alterations and ASA II is a patient with mild systemic disease but no functional limitations. Nevertheless, the Spanish Society of Anesthesiology, Resuscitation and Pain Management (SEDAR) contradicts the recommendations of the European Society of Gastroenterologists on the administration of propofol by non-anesthetists, claiming that this procedure is unsafe 6. As indicated by the European Society of Gastrointestinal Endoscopy (ESGE) and the European Society of Gastroenterology and Endoscopy Nurses and Associates (ESGENA), this decision would appear to be more influenced by political and economic reasons than by the available evidence. Therefore, there is a need for anesthetists to intervene in this respect, in order to improve the provision of sedation in endoscopy units 7.
A growing number of medical units are implementing propofol sedation, as many endoscopies are performed in low-risk patients. Moreover, the use of propofol is probably more efficient, since the induction and recovery time is shorter than with benzodiazepines and opiates 8. However, in order to guarantee the quality of the sedation procedure when performed by non-anesthetists, the personnel involved and the endoscopy unit where the procedure is performed must comply with the latest recommendations issued in this regard by the corresponding scientific societies. These guidelines refer to the provision of specific, appropriate training and to the allocation of material resources to facilitate the correct implementation of the procedure 5,9. With regard to training, there is a broad consensus on the contents required, namely prior assessment of risk and of the pharmacology of anesthetic agents, intra-procedural monitoring, early detection and control of adverse events, post-procedure care and training in advanced life support. Moreover, after knowledge acquisition, these skills must be demonstrated in a real-world scenario, together with the necessary technical skills for the management of possible complications that arise from sedation 10. At present, SEED-endorsed training courses in these skills are available, thus accrediting the capability of a clinical professional to undertake this activity 11.
The main aim of this study was to evaluate the quality of the sedation procedure administered by non-anesthetists in a digestive endoscopy unit one year after its introduction. The secondary goals were to identify the number of sedation procedures performed with propofol by non-anesthetist personnel, to record the incidence and types of adverse events, to determine the proportion of staff in the unit who adhered to the recommendations for the procedure and to determine the level of completion of the clinical records related to sedation.
METHOD
In 2014, a medical team consisting of a nurse and an endoscopist completed the SEED-approved training course for the administration of propofol in digestive endoscopy. Subsequently, in collaboration with the Anesthesia Service of the Costa del Sol Hospital in Marbella, a training program was designed for all the endoscopists and nurses in the unit. This program consisted of a 100-hour course divided into 80 non-contact hours (virtual platform) and 20 contact hours with theoretical training (six hours) and practical training (14 hours), in accordance with the training requirements set out in the consensus documents 12. The theoretical module addressed diagnostic and therapeutic digestive endoscopy, the legal framework for sedation by non-anesthetists, clinical guidelines for sedation in digestive endoscopy, basic anesthesiology, the pharmacology of anesthetic agents, patient monitoring, airway management, post-sedation controls, the prevention and treatment of complications, the organization and management of the endoscopy unit and sedation by bolus administration and infusion pumps. The practical module consisted of 14 hours of real-world hands-on experience supervised by anesthetists. In addition, these medical professionals took an accredited advanced life support course. Those who successfully completed the training process received a diploma that accredited their qualification for the management of propofol sedation in patients classed as ASA I-II.
The material and human resources proposed by the SEED 9 were employed to implement the anesthetic procedure. An extra nurse was present in the recovery area to support the examination room staff in the performance of therapeutic procedures (endoscopic retrograde cholangiopancreatography [ERCP], echoendoscopies, colonoscopies and therapeutic gastroscopies) since propofol sedation in simple procedures is carried out by the same personnel that perform the clinical examination. During the examinations, an auxiliary nurse was present in addition to the endoscopist-nurse team, to assist with basic tasks and to collaborate in the colonoscopy when necessary. Further electro-medical equipment was acquired for patient monitoring, including two target-controlled infusion pumps and an additional cardiopulmonary resuscitation cart. A specific sedation procedure was developed for endoscopy in collaboration with the Anesthesia Service, which included the design and management of the entire process, provision of the necessary materials and stipulation of the functions of the medical staff involved. In addition, a checklist was created to ensure the unambiguous identification of the patient, record the results of the pre-sedation evaluation, control the monitoring of vital signs and to note the medication administered and the post-anesthetic evaluation, among other aspects. The Patient Safety Observatory of the Andalusian regional government (Junta de Andalucía) recognizes the use of this checklist as contributing to patient safety 13.
The procedure was first implemented in January 2016 in the Digestive Endoscopy Unit of the Costa del Sol Hospital (Marbella, Spain). For the next twelve months, all patients who underwent an endoscopic procedure with superficial sedation (benzodiazepines and/or opioids) or deep sedation (propofol) were followed-up prospectively. Cardiorespiratory complications (hypotension, hypoxemia, arrhythmias and bronchopulmonary aspiration) and more serious events that might require intubation or result in the death of the patient were classed as adverse events. The research team adopted the parameters proposed by the ASGE 14, classifying oxygen saturation < 85% as hypoxia, blood pressure < 90/50 mmHg or > 20% below the baseline as hypotension and blood pressure > 190/130 mmHg or > 20% above the baseline as hypertension.
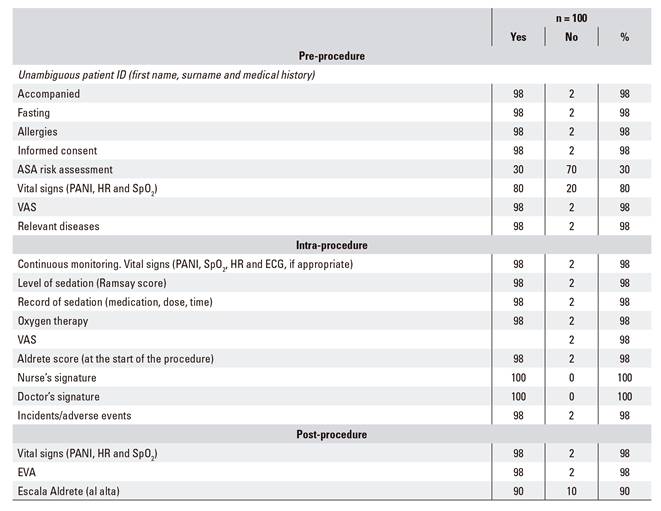
To ascertain the quality of the clinical records obtained, a random audit was performed of 100 clinical records of patients who had been sedated with propofol by non-anesthetists during the study period. The degree of compliance with each of the checklist items regarding the quality of the sedation procedure were determined. These items included the unequivocal identification of the patient, performance of the pre-procedural evaluation (fasting, informed consent, pain, constant signs, allergies, etc.), ASA risk assessment, medication record (type of drug administered, time and dose), continuous monitoring of the patients' condition (oxygen saturation [SpO2], non-invasive blood pressure [NIBP] and heart rate [HR], plus electrocardiogram [ECG] for patients with a history of heart disease), post-procedural evaluation (pain and vital signs), evaluation of incidents and/or adverse events, evaluation of compliance with discharge criteria (pain, vital signs and Aldrete score) and the signatures of the nurse or doctor.
The staff in the unit were not informed that this study was being conducted in order to avoid a possible bias in the results, since their clinical practice might vary if they knew that they were under evaluation. In order to randomize the clinical records and thus avoid a possible selection bias, the archive and documentation unit was asked to select the case histories of 50 patients whose final clinical history digit was an even number and another 50 case histories whose final clinical history digit was an odd number. Indicators were considered to be of an acceptable quality when the adherence to each of the aspects evaluated was 80% or greater. All patients gave their informed consent for sedation with propofol. Data protection laws with respect to patient information were complied with at all times.
RESULTS
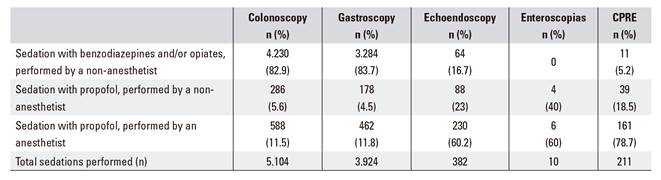
During 2016, a total of 9,631 endoscopic procedures were performed with superficial or deep sedation. These procedures were mainly colonoscopies (53%) and gastroscopies (40.7%), followed by echo-endoscopies (4%), ERCP (2.2%) and enteroscopies (0.1%). Of these patients, 7,589 (78.8%) were sedated by non-anesthetists using benzodiazepines and/or opiates, 595 (6.2%) by non-anesthetists using propofol and 1,447 (15%) by anesthetists using propofol. Table 1 details the sedation used according to the endoscopic procedure performed and the professional category of the medical staff involved.
Table 1 Type of sedation used according to the endoscopic procedure performed and the professional category of the medical staff involved
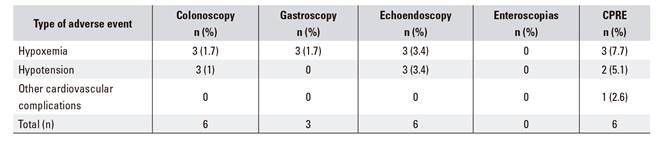
A total of 507 patients received propofol sedation administered by non-anesthetists during the study period, 274 (54%) were male and 233 (46%) were female. The average age within both groups was 64 years. During sedation, twelve patients (2.4%) had adverse events. One case was an episode of hypoxemia, which was resolved with a mandibular traction and increased oxygen flow using nasal glasses. In addition, eight patients suffered episodes of mild hypotension that improved spontaneously with fluid therapy. One patient suffered a hypertensive crisis accompanied by an arrhythmia that required specific endovenous treatment and the suspension of the endoscopic procedure until hemodynamic stability was regained. None of the patients needed artificial ventilator support or endotracheal intubation. Table 2 details the adverse events that occurred during propofol sedation performed by non-anesthetists, according to the endoscopic procedure used.
Table 3 shows the degree of compliance with the recommended criteria in relation to the completion of patient records. All the aspects evaluated had scores of 80% or higher, except for adherence to ASA risk assessment.
Table 3 Compliance with recommended criteria for the completion of patient records
SpO2: oxygen saturation; NIBP: non-invasive blood pressure; HR: heart rate; ECG: electrocardiogram.
All eight of the nursing professionals who had been trained to administer propofol adhered to the procedure, in accordance with the indications made by the endoscopist. By contrast, only 17 of the physicians who had been trained (18%) modified their usual practice and adhered to the new procedure.
DISCUSSION
Studies show that the administration of propofol by non-anesthetists is feasible and safe, provided that it is given to appropriately selected patients 15,16,17. Although propofol offers the patient a faster recovery than midazolam or fentanyl 18, the usual practice is to perform endoscopic procedures with superficial sedation using benzodiazepines and/or opioids. Only a small percentage of endoscopists in our study chose to apply sedation with propofol, which could be due to conflicts of interest, uncertainty regarding the correct handling of the drug, professional prejudice or even fear of legal repercussions from anesthetists 7. On the contrary, acceptance is high among nursing professionals; this may reflect the legal backing given to the administration of this drug under medical instructions and the assumption that this is a standardized nursing intervention.
The observed incidence of adverse events in the case of cardiovascular complications, mainly hypoxia and hypotension, corroborates the findings of previous studies based on similar populations 19. Although there is no universally recognized threshold for the definition of hypoxia, hypotension or hypertension in digestive endoscopy, our research team chose to use the definitions proposed by the ASGE 14. Our data coincide with those of an American study which observed higher rates of hypoxia in ERCP and lower rates in gastroscopies and colonoscopies 19. Although the results obtained are satisfactory, the sample size is a limitation and future studies with a larger cohort are needed to establish definitive conclusions about the safety of propofol sedation by non-anesthetists in ASA I-II patients.
One of the main limitations of the present study is that no analysis was made of the adverse events associated with sedation performed with the usual practice, in order to compare them with the events identified when propofol is administered by non-anesthetists. Such an analysis might provide the motivation to medical professionals who are reticent to adopt the latter procedure, provided that the findings are consistent with those of a recent meta-analysis. This study showed that propofol is associated with the same proportion of hypoxia and hypotension episodes as traditional sedatives, while there is a slightly lower probability of complications with propofol in non-advanced endoscopies 20.
Furthermore, patients who are sedated with propofol tend to express a greater satisfaction with the procedure 8,21,22,23. Accordingly, future studies should be undertaken to compare patient satisfaction when receiving sedation with propofol in comparison with benzodiazepines and/or opiates. If there is a greater satisfaction with propofol, this would provide further motivation to encourage the adoption of this procedure.
With regard to the quality of clinical record keeping, our study shows that, in general, medical professionals comply with recommendations for each of the aspects considered, except for the evaluation of the ASA risk prior to the endoscopic procedure, for which adherence is very low. This shortcoming could be due to the fact that patients have already been evaluated in the clinic when they are referred to this unit. Therefore, professionals may not consider it necessary to repeat the evaluation. Our findings reflect the importance of auditing clinical records as relevant elements aimed to improve healthcare practices 24, as the lack of adherence to ASA risk assessment prior to anesthesia can provoke a serious increase in the rate of complications and adverse events 25,26.
CONCLUSIONS
Our study results suggest that propofol can be administered safely and effectively by a qualified endoscopist-nurse team in patients with ASA I-II risk. Audits of adherence to the recommended procedure facilitate the identification of areas for improvement and encourage further efforts in aspects that have not yet been consolidated.
BIBLIOGRAFÍA
1. Goudra BG, Singh PM, Gouda G, et al. Safety of non-anesthesia provider-administered propofol (NAAP) sedation in advanced gastrointestinal endoscopic procedures: comparative meta-analysis of pooled results. Dig Dis Sci 2015;60(9):2612-27. [ Links ]
2. Gouda B, Gouda G, Borle A, et al. Safety of non-anesthesia provider administered propofol sedation in non-advanced gastrointestinal endoscopic procedures: a meta-analysis. Saudi J Gastroenterol 2017;23(3):133-43. [ Links ]
3. Lee JK, Jang DK, Kim WH, et al. Safety of non-anesthesiologist administration of propofol for gastrointestinal endoscopy. Korean J Gastroenterol 2017;69(1):55-8. [ Links ]
4. Vargo JJ, Niklewski PJ, Williams JL, et al. Patient safety during sedation by anesthesia professionals during routine upper endoscopy and colonoscopy: an analysis of 1.38 million procedures. Gastrointest Endosc 2017;85(1):101-8. [ Links ]
5. Igea F, Casellas JA, González-Huix F, et al. Sedación en endoscopia digestiva. Guía de práctica clínica de la Sociedad Española de Endoscopia Digestiva. Rev Esp Enferm Dig 2014;106:195-211. [ Links ]
6. Pelosi P, Board of the European Society of Anaesthesiology. Retraction of endorsement: European Society of Gastrointestinal Endoscopy, European Society of Gastroenterology and Endoscopy Nurses and Associates and the European Society of Anaesthesiology Guideline - Non-anaesthesiologist administration of propofol for gastrointestinal endoscopy. Eur J Anaesthesiol 2012;29(4):208-9. DOI: 10.1097/EJA.0b013e32834f5e5a [ Links ]
7. Pérez-Cuadrado E, González A, Lancho A, et al. Safety and risk factors for difficult endoscopist-directed ERCP sedation in daily practice: a hospital-based case-control study. Rev Esp Enferm Dig 2016;108(5):240-5. [ Links ]
8. McQuaid KR, Laine L. A systematic review and meta-analysis of randomized, controlled trials of moderate sedation for routine endoscopic procedures. Gastrointest Endosc 2008;67(6):910-23. [ Links ]
9. Dumonceau J-M, Riphaus A, Beilenhoff U, et al. European curriculum for sedation training in gastrointestinal endoscopy: position statement of the European Society of Gastrointestinal Endoscopy (ESGE) and European Society of Gastroenterology and Endoscopy Nurses and Associates (ESGENA). Endoscopy 2013;45(06):496-504. [ Links ]
10. Vargo JJ, DeLegge MH, Feld AD, et al. Multisociety sedation curriculum for gastrointestinal endoscopy. Gastroenterology 2012;143(1):e18-41. DOI: 10.1016/j.gie.2012.03.001 [ Links ]
11. Sociedad Española de Endoscopia Digestiva, SEED. Cursos de Sedación 2017. Citado 24 de septiembre de 2017. Disponible en: http://www.wseed.org/index.php/cursos-y-congresos/cursos-de-la-seed/cursos-de-sedacion/curso-de-sedacion-2017 [ Links ]
12. Consenso sedación endoscopia. Citado 24 de septiembre de 2017. Disponible en: http://www.aegastro.es/sites/default/files/archivos/MBE-CC/consenso_sedacion_endoscopia_aeg.pdf [ Links ]
13. Listado verificación endoscopias Costa del Sol. Citado 24 de septiembre de 2017. Disponible en: http://www.juntadeandalucia.es/agenciadecalidadsanitaria/observatorioseguridadpaciente/gestor/sites/PortalObservatorio/cirugiasegura/documentos/listado_verificacion_endoscopias_costa_sol.pdf [ Links ]
14. Cotton PB, Eisen GM, Aabakken L, et al. A lexicon for endoscopic adverse events: report of an ASGE workshop. Gastrointest Endosc 2010;71(3): 446-54. [ Links ]
15. Sieg A, bng-Study-Group, Beck S, et al. Safety analysis of endoscopist-directed propofol sedation: a prospective, national multicenter study of 24?441 patients in German outpatient practices. J Gastroenterol Hepatol 2014;29(3):517-23. [ Links ]
16. Rex DK, Deenadayalu VP, Eid E, et al. Endoscopist-directed administration of propofol: a worldwide safety experience. Gastroenterology 2009;137(4):1229-37;quiz 1518-9. [ Links ]
17. Sathananthan D, Young E, Nind G, et al. Assessing the safety of physician-directed nurse-administered propofol sedation in low-risk patients undergoing endoscopy and colonoscopy. Endosc Int Open 2017;5(2):E110-5. DOI: 10.1055/s-0042-121667 [ Links ]
18. Vargo JJ, Zuccaro G, Dumot JA, et al. Gastroenterologist-administered propofol versus meperidine and midazolam for advanced upper endoscopy: a prospective, randomized trial. Gastroenterology 2002;123(1):8-16. [ Links ]
19. Sharma VK, Nguyen CC, Crowell MD, et al. A national study of cardiopulmonary unplanned events after GI endoscopy. Gastrointest Endosc 2007;66(1):27-34. [ Links ]
20. Wadhwa V, Issa D, Garg S, et al. Similar risk of cardiopulmonary adverse events between propofol and traditional anesthesia for gastrointestinal endoscopy: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2017;15(2):194-206. DOI: 10.1016/j.cgh.2016.07.013 [ Links ]
21. Lera dos Santos ME, Maluf-Filho F, Chaves DM, et al. Deep sedation during gastrointestinal endoscopy: propofol-fentanyl and midazolam-fentanyl regimens. World J Gastroenterol 2013;19(22):3439-46. [ Links ]
22. Levitzky BE, López R, Dumot JA, et al. Moderate sedation for elective upper endoscopy with balanced propofol versus fentanyl and midazolam alone: a randomized clinical trial. Endoscopy 2012;44(1):13-20. [ Links ]
23. Dewitt J, McGreevy K, Sherman S, et al. Nurse-administered propofol sedation compared with midazolam and meperidine for EUS: a prospective, randomized trial. Gastrointest Endosc 2008;68(3):499-509. [ Links ]
24. American Association for Study of Liver Diseases, American College of Gastroenterology, American Gastroenterological Association Institute, et al. Multisociety sedation curriculum for gastrointestinal endoscopy. Gastrointest Endosc 2012;76(1):e1-25. [ Links ]
25. Zakeri N, Coda S, Webster S, et al. Risk factors for endoscopic sedation reversal events: a five-year retrospective study. Frontline Gastroenterol 2015;6(4):270-7. [ Links ]
26. Heuss LT, Schnieper P, Drewe J, et al. Risk stratification and safe administration of propofol by registered nurses supervised by the gastroenterologist: a prospective observational study of more than 2000 cases. Gastrointest Endosc 2003;57(6):664-71. [ Links ]
Received: October 09, 2017; Accepted: January 15, 2018











 texto em
texto em