Meu SciELO
Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Revista Española de Enfermedades Digestivas
versão impressa ISSN 1130-0108
Rev. esp. enferm. dig. vol.110 no.5 Madrid Mai. 2018
https://dx.doi.org/10.17235/reed.2018.5040/2017
ORIGINAL PAPERS
Endoscopic ultrasound-guided choledochoduodenostomy after a failed or impossible ERCP
1Servicio de Aparato Digestivo. Complejo Asistencial Universitario de Salamanca. Instituto de Investigación Biomédica de Salamanca (IBSAL). Salamanca, España
INTRODUCTION
Jaundice is a very common problem in daily clinical practice and may have different etiologies. There are differences between pre-hepatic (hemolysis, congenital disorders, etc.), hepatic (toxic, viral, etc.) and post-hepatic hyperbilirubinemia (obstructive). The causes of obstructive jaundice can be benign, such as choledocholithiasis and postoperative stenosis, or malignant, such as cholangiocarcinoma and pancreatic neoplasms. Most patients with malignant biliary obstruction are diagnosed at an advanced stage and are not candidates for surgery with a curative intent 1. Regardless of the etiology, drainage of the bile duct is the priority of palliative treatment for the resolution of jaundice and an improvement in the quality of life. Previously, the only available treatment was invasive surgery, with elevated morbidity and mortality rates and a longer in-hospital stay. Currently, endoscopic retrograde cholangiopancreatography (ERCP) is the method of choice for the treatment of biliopancreatic pathologies, particularly in patients with malignant obstruction of the bile duct and cases with stent placement as a first palliative measure. However, this procedure cannot be performed in 5-10% of cases due to a failure in the cannulation of the bile duct or tumor infiltration (duodenal obstruction), which limits access to the bile duct. In these cases, alternative decompression methods are required 2,3,4. Percutaneous trans-hepatic biliary drainage (PTBD) is an effective alternative but with important morbidity and mortality rates and a complication risk (bile leak, peritonitis, hemorrhage) of up to 30%. Sometimes external biliary drainage is required, with a significant impact on patient quality of life 2.
Another alternative method to ERCP is endoscopic ultrasound-guided biliary drainage (EUS-BD). This procedure alleviates jaundice secondary to a confirmed distal biliary obstruction, with a benign or malignant etiology 4. This method is based on the combination of three possible routes of access (intrahepatic biliary access, extrahepatic access and access via the pancreatic ducts) and three drainage routes (transmural, direct transpapillary and retrograde transpapillary drainage) 5. This procedure was described for the first time in 2001 by Giovannini et al. 6. The endoscopic ultrasound-guided derivation or anastomosis between the middle bile duct and the duodenum (EUS-guided choledochoduodenostomy, EUCD) is the most commonly used technique. Together with EUS-guided hepaticogastrostomy (EUS-HG), they represent a useful alternative in these types of cases 2. The bile duct is more exposed to endoscopic ultrasound than the intrahepatic ducts and it is easier to approach when dilated. Thus, it is possible to plan a clean, easy and fast approach that minimizes risks, particularly in the case of patients with ascites, in which a percutaneous approach is dangerous. In addition, the position of the endoscope on the duodenal bulb is more stable and firm than when positioned on the medial part of the stomach in order to puncture the biliary duct, and EUS-HG also requires a dilated intrahepatic biliary duct. Both techniques may be an option in cases of distal obstruction of the bile duct. However, when the obstruction is in the hepatic hilum, drainage via the stomach is preferred. Both techniques have complication rates of up to 16% 2, including bile leakage, bilioma, duodenal perforation, hemorrhage, severe septic problems and death in some cases. Therefore, a wide experience of biliary endoscopy (ERCP) and therapeutic endoscopic ultrasound is required.
The choice of technique depends on the availability in each center and the experience of the medical practitioners. It is also necessary to take into account the disadvantages of each procedure and the impact on the quality of life of the patient. Most patients with malignant biliary obstruction are diagnosed in an advanced stage and are not candidates for surgery with a curative intent 1. These cases may have jaundice, itching, anorexia, malnutrition, cholangitis and, finally, a premature death. Relief of the biliary obstruction is essential to resolve the jaundice, improve quality of life and increase survival rates.
The following describes six clinical cases where the EUCD procedure was performed after a failed or impossible ERCP. We present our experience during the period from 2011 to 2015.
METHODS
The study population included six clinical cases who underwent endoscopic ultrasonography-guided choledochoduodenostomy (EUCD) after a failed or impossible ERCP. The study period was from November 2011 to December 2015. The clinical and endoscopic characteristics of the patients are shown in table 1.
Patients were assessed in our department during admission. The cases were included in the study due to the following symptoms: obstructive jaundice, abdominal pain and alteration in the liver function tests (in all six cases). This was associated with constitutional symptoms in two cases. The first examination was an abdominal ultrasound followed by a CT scan, which revealed findings compatible with obstruction of the biliary duct caused by a tumor.
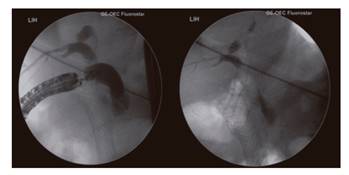
The therapeutic procedure was carried out in the endoscopy room, with two endoscopists and qualified nurses. A deep sedation was used in all cases with a 1% propofol infusion, without orotracheal intubation, associated with oxygen therapy and non-invasive monitoring. At first, ERCP was attempted for biliary drainage, which could not be completed in one case. The papilla could not be reached in the other five cases. A decision was made to perform EUS-guided drainage on the same day in three of the cases and was scheduled for a later date in the other three cases. EUS was performed with the sectorial-linear ultrasound echoendoscope Olympus(r) GF-UCT140 (Fig. 1) and the duodenal bulb was reached in order to localize and visualize the dilated bile duct and localize and characterize the type of stenosis. CO2 was not available during the examination.
The endoscopist attempted a direct transduodenal approach. The site of puncture was determined with the ultrasound endoscope, and color Doppler ultrasound was used to prevent the vessels from interfering with the operating field. Access to the bile duct was obtained from the duodenal bulb via a puncture with a 19G needle from Expect(tm) Boston Scientific(r). In order to confirm the location, the biliary contents were aspirated and a cholangiography was performed after contrast injection. Subsequently, a 0.035" probe was introduced towards the proximal biliary area with the use of the needle and the position was secured in the bile duct. After removing the EUS needle over the probe, the duodenal puncture and bile duct orifice were expanded with a Boston(r) pre-cut sphincterotome in two cases and a 6Fr Endo-flex(r) cystotome in four cases, as the cystotome was not available in all cases. Subsequently, the duodenum-bile duct segment was dilated with a biliary balloon (Boston Scientific(r) Hurricane(tm) RX 6mm x 40 mm) in five cases and with a pyloric balloon (Boston Scientific CRE(tm) PRO Wireguided 55 mm x 6 mm) in one case. Then, a 10 x 60 mm fully covered self-expanding metal prosthesis (Walflex(tm) Biliary Rx Stent-Boston Scientific(r)) was placed through the probe in order to establish the transmural route or cross through the stenosis, and the bile output was verified (Fig. 2). The procedure was guided at all times via radiology, ultrasound endoscopy and a direct endoscopic view. The dilation and placement of the metal stent have the highest risk of complications. The patient was referred to the hospitalization unit after the procedure.
RESULTS
Six patients were included in the study, three males and three females. The average age at the time of the procedure was 63 years (range: 43-79 years). All the biliary obstructions were caused by a tumor and were not secondary to surgery. Three were secondary to a neoplasm in the head of the pancreas and three had a duodenal origin. The extrahepatic bile duct was dilated in all cases, with an average bile duct size of 13.36 mm. EUS-guided drainage was performed in one case due to a failed cannulation of the bile duct as the papilla could not be accessed. The procedure was performed in the remaining five cases due to duodenum stenosis and the fact that it was impossible to access the biliary drainage. A metal duodenal stent was used in these cases. The duodenal stent was placed prior to the biliary drainage in one case and after the procedure in the remaining cases; two during the same operation and in the other two at a later stage. A Wallflex(tm) Duodenal Stent 22 x 90 mm Boston Scientific(r) prosthesis was used. The stents did not contribute to the dysfunction of the drainage in any of the cases. All the patients had obstructive jaundice, with an average total bilirubin level of 10.75 mg/dl. The analysis identified alkaline phosphatase levels at 1,077 U/l, gamma-glutamyl-transferase at 2,022 U/l, and leukocytes at 12,790/µl.
Sedation was applied with propofol (with continuous infusion) in all cases, which was administered by an expert endoscopist and qualified nurse with an average dose of 770 mg (310-1,070 mg) per examination. The anesthetic risk was assessed and four patients were classified as ASA III and two as ASA II. Antibiotic therapy was administered in all cases. Two cases were a prophylactic single dose of intravenous amoxicillin/clavulanate prior to the procedure and the remaining four patients were already undergoing antibiotic therapy with intravenous piperacillin/tazobactam at 4 g/0.5 g every six hours due to secondary acute cholangitis.
Two of the six procedures could not be completed due to a technical failure. It was impossible to insert the stent as the probe became displaced, which meant that PTBD was required for endoprosthesis placement. There were no severe early complications and there was one case of pneumoperitoneum after the procedure which had a good evolution and did not require emergency surgery. With regard to the four successful cases, two had late complications and required a replacement due to stent migration. There were technical difficulties in one case that hindered a proper placement of the stent and EUS was performed due to the persistence of high levels of total bilirubin seven days after the procedure. This showed that the stent had migrated and a new one was placed without further complications. With regard to the remaining case, the patient presented symptoms of acute cholangitis 14 months after the procedure. EUS revealed a migration of the stent and a new one was placed.
An improvement in the analysis of the results of all patients was achieved, with a decrease in the total levels of bilirubin and cholestasis. Four of the patients died due to progression of the tumor, with an average monitoring period of six months after the operation (range 1-16 months).
DISCUSSION
Patients with biliary stenosis due to a malignant cause have a poor short-term prognosis, as most are already in an advanced stage of the disease at diagnosis. This limits the therapeutic options with a curative intent and these cases are candidates for palliative care only. In addition, they present signs and symptoms that lead to a fast and significant deterioration of their general health, such as obstructive jaundice, cholangitis, itching, duodenal obstruction, etc. Currently, non-surgical procedures are the treatment of choice to drain the bile duct in malignant unresectable pathologies. When ERCP cannot be performed as a bile duct cannulation is not possible or due to a malignant obstruction that prevents access to the papilla, more invasive procedures such as PTBD and EUS-BD are required. We propose the use of a therapeutic decision algorithm in these cases, in which the choice depends on experience with EUS-BD or the possibility to refer patients to endoscopy units with a wide experience in this sort of drainage technique (Fig. 3).
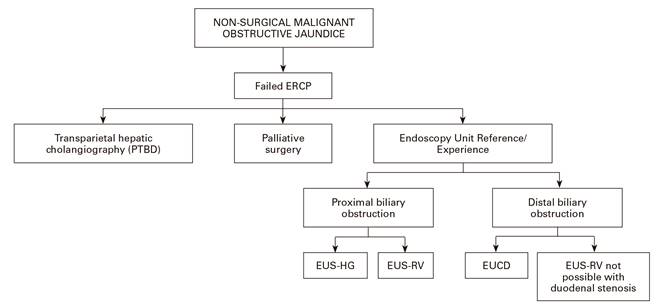
Fig. 3 Therapeutic decision algorithm for non-surgical malignant obstructive jaundice (EUS-HG: hepatico-gastrostomy. EUS-RV: rendez-vous. EUCD: choledochoduodenostomy).
PTBD had been considered as the main therapeutic choice after a failed ERCP for several decades. Khashab et al. 7 compared EUCD and PTBD with regard to safety, effectiveness and cost. This study included 73 patients who underwent EUCD (n = 22) and PTBD (n = 51) after a failed ERCP. Although the technical success rate was higher in the PTBD group (100% vs 86.4%, p = 0.007), clinical success was similar in both groups (92.2% vs 86.4%, p = 0.40). The authors concluded that both procedures are comparatively effective in the treatment of malignant distal obstruction of the bile duct. However, EUCD had a lower rate of adverse events and a lower cost, due to the need for fewer reintervention procedures. These results suggest that EUCD should be the technique of choice after a failed ERCP in experienced centers.
Over the last 20 years, EUS has evolved from a purely diagnostic tool to a therapeutic technique. Some of the different types of EUS-guided therapies include FNAP, drainage of pancreatic cysts and drainage of the bile duct (EUS-BD). EUS-BD makes the visualization of the biliary obstruction possible and allows access to the bile duct from the gastrointestinal lumen. The process varies according to the access approach and the drainage route used (Fig. 4). The choice of one technique over another depends on the anatomy and the origin of the obstruction 2.

Fig. 4 Access routes of EUS-BD: intrahepatic (1, 3) and extrahepatic (2, 4). Transmural biliary drainage (1, 2), hepatico-gastrostomy (1) or choledochoduodenostomy. Transpapillary access (3, 4) enables retrograde access via rendez-vous (2, 4). Obtained from Pérez-Miranda et al. Curr Gastroenterol Rep, 2013.
Wiersema et al. 8 described for the first time in 1996 EUS-guided biliary drainage via ERCP as an emerging technique which may be an alternative to PTBD and surgery. Other studies 1,2 have assessed the effectiveness and safety of EUS-guided drainage, as well as its impact on the quality of life of patients with inoperable disease due to the advanced stage of their condition. Although current data may be limited, EUS-guided biliary drainage is a safe alternative to surgery in patients with bile duct obstruction and a failed or impossible ERCP 3,7,9.
In 2004, Mallery et al. 10 introduced the technique of EUS-guided rendez-vous (EUS-RV) into the field for biliary drainage via conventional ERCP. The advantage of the retrograde approach is the possibility to drain the structure in the same therapeutic procedure when the papilla can be accessed endoscopically, although malignant obstructions can make access difficult. EUCD is recommended over EUS-RV in the case of malignant obstructions and EUS-RV is a better option in patients with benign obstructions 4.
EUS-HG has a clinical benefit in cases of obstruction of the duodenal bulb. Takada et al. 1 did not report significant differences with regard to the success and complication rates between EUCD and EUS-HG drainage. The former option is technically simpler and more accessible. Artifon et al. 2) compared both techniques and their success and complication rates in patients with malignant distal biliary obstruction. Both of them had a high success rate and there were no statistically significant differences.
There is no standard with regard to the use of one technique or route over another and it largely depends on the endoscopist and their experience. Some studies 2 recommend EUS-HG when a double drainage (biliary and duodenal) is required. However, EUCD could also be performed in these cases with a lower complication rate. The choice of the approach to the bile duct depends on the location of the obstruction in 80% of cases and on the endoscopist in the remaining 20%. Therefore, drainage will be transgastric in hilar or proximal obstructions of the bile duct, whereas it may be transduodenal or transgastric in distal obstructions.
Ever since it was described by Giovannini et al. in 2001 6, EUCD has become an effective rescue procedure. Many studies report a high success rate of over 90% and a complication rate related to the procedure of approximately 19%. EUCD is a complex technique and it should be performed by an endoscopist with training in EUS and ERCP in order to minimize the complications derived from this procedure as much as possible. Hara K et al. 4 recommend a learning process guided by an expert endoscopist, at least during the first 20 procedures. EUS-BD is considered to be a minimally invasive technique, even in patients with anatomical alterations such as partial gastrectomies or Billroth-type reconstructions. This makes it possible to perform the entire operation in a single endoscopic procedure and, therefore, is safer. Other advantages include the possibility to obtain color Doppler information, a lower rate of complications associated with the procedure and a better quality of life as the patient does not require external drainage 2,3. In spite of its increased use over the last several years, the tools involved need to be improved to include more convenient and safe devices. Hara et al. 4 and Ogura et al. 5 provide a general review of the EUS-guided biliary drainage technique, as well as of the different devices used in the procedure, the selection of prostheses and candidate patients.
According to published reports 5,11,12, the rate of complications and adverse effects in EUCD is an estimated 16%, including bile leakage, hemorrhage, pneumoperitoneum, infection (peritonitis, cholangitis, cholecystitis) or stent migration. Two deaths were recorded in the series of Maluf-Filho et al. 13, one due to cardiogenic shock and the other due to a bile leak. Non-covered metal prostheses were used in both cases. A large multi-center study from Spain 14 described the reasons behind technical failures in this procedure, the most common being the difficulty to manipulate the probe (impossible to perform an intraductal insertion) (20/41), followed by the loss of its position and migration. The influence of different factors on the success and complication rates, such as the patient, experience of the endoscopist and type of hospital, was also analyzed.
A recent systematic analysis 9 assessed the effectiveness and safety of EUCD and the transduodenal (TD) and transgastric (TG) approaches. This included 42 studies with 1,192 patients. The TD and TG approaches were assessed comparatively and there were no significant differences with regard to the cumulative technical success rate, functional success rate or the rate of adverse events between both techniques or when compared to EUCD. The analysis concluded that EUCD is an effective alternative procedure even though it is associated with a significant morbidity. A Japanese multi-center study published by Kawakubo et al. 15 in 2014 also recorded technical success and complication rates similar to those of other series. Better results were obtained when using covered metallic stents, with a lower rate of bile leakage (4%) with metal stents compared to plastic stents (11%).
The most worrying concern apart from stent placement in the EUCD procedure is the possibility of a bile leak to the peritoneum or retroperitoneum, which would require surgery in most cases. Theoretically, this might be avoided with the use of fully covered self-expanding metal stents 3 or new devices. In this series, no early severe complications were reported (death, surgery or sepsis). There was one case of pneumoperitoneum after the procedure which required the placement of an endoprosthesis with interventional radiology. This was resolved and did not require emergency surgery. Among the successful cases, two presented late complications and required a stent replacement due to migration. The literature reports a complication rate associated with PTBD of 10% to 21-32% 2, including hemorrhage, cutaneous fistula, cholangitis, peritonitis and empyema 1. There are also reports of a longer in-hospital stay and deaths associated with this procedure, of an estimated 0-5.6% 2. In our series, two patients required PTBD and endoprosthesis placement, and there were no other complication.
The type of prosthesis used in the procedure is a crucial element 1,3. It is known that covered metal stents cause fewer complications than plastic ones. However, there is no consensus regarding the best metal prosthesis available. The main complications of fully-covered stents are frequent migrations. Takada et al. 1 describe the first case of HG with a fully covered metal stent, with an improvement in symptoms and analysis results after three months and no complications. Park et al. 16 assessed the short-term complication and effectiveness rates of fully covered metal stents with an anchoring flap in a multi-center prospective study, and a lower rate of stent migration was observed. Other prostheses, such as Niti-S Nagi(r) Stent Taewoong Medical 17, have also shown good results in endoscopic cholecystogastrostomy with adequate anchoring and low migration rates. Moon et al. 18 used fully covered metal stent for lumen apposition (Niti-S Spaxus(r) Taewoong Medical) in endoscopy-guided drainage of a peripancreatic fluid collection and acute cholecystitis. Good results were obtained, although it was a retrospective study with a small case series. Recently, Kunda et al. 3 reported their experience with EUCD and the use of the AXIOS(tm) and Hot AXIOS(tm) self-expandable metal stents. These new devices make it possible to perform a fistula from the duodenal/gastric wall to the biliary wall with electrocautery and to place a lumen-apposing stent in a single procedure (Hot AXIOS(tm)), thus preventing the use of the cystotome or any other additional devices. The authors reported a success rate of 98.2% for this procedure and stent migration in 9.3% of the patients during follow-up. These devices can automatically seal the space between the stent and the fistula walls due to their expanding capacity. They also minimize the time from the creation of the fistula between the duodenum and the bile duct and the stent placement.
There are no studies or case series comparing the different types of stents. In our series we used fully covered biliary metal stents (Wallflex(tm) Biliary RX Fully Covered Stent System Boston Scientific(r)) in all cases, which are also used in most series. Some of the advantages include their self-expanding nature, which can reduce the risk of a bile leak, and their wide diameter, which provides a longer duration and lower migration rates. In addition, it is possible to place another plastic prosthesis intra-stent, particularly in cases of intra-stent tumor growth. Their main disadvantage is the high cost.
We can conclude that, in spite of the small case series, this study showed good results of the EUCD technique, which is becoming a valid therapeutic choice in some selected cases. EUCD has been shown to be an effective alternative to PTBD or surgery in the case of a failed ERCP for the treatment of malignant obstruction of the bile duct. With the increasing experience of practitioners and the technical advances in the field, the safety of this procedure is likely to improve. Different case series, retrospective studies and case reports of EUCD have been reported with satisfactory and promising results. More prospective studies are required with homogeneous inclusion criteria and a long-term follow-up which would allow a set of criteria to be established and advance this technique in the field of interventional endoscopy.
BIBLIOGRAFÍA
1. Takada J, Carmo AM, Artifon EL. EUS-guided biliary drainage for malignant biliary obstruction in patients with failed ERCP. J Interv Gastroenterol 2013;3(3):76-81. DOI: 10.7178/jig.128 [ Links ]
2. Artifon EL, Marson FP, Gaidhane M, et al. Hepaticogastrostomy or choledochoduodenostomy for distal malignant biliary obstruction after failed ERCP: Is there any difference? Gastrointest Endosc 2015;81(4):950-9. [ Links ]
3. Kunda R, Pérez-Miranda M, Will U, et al. EUS-guided choledochoduodenostomy for malignant distal biliary obstruction using a lumen-apposing fully covered metal stent after failed ERCP. Surg Endosc 2016;30(11):5002-8. DOI: 10.1007/s00464-016-4845-6 [ Links ]
4. Hara K, Yamao K, Mizuno N, et al. Endoscopic ultrasonography-guided biliary drainage: Who, when, which, and how? World J Gastroenterol 2016;22(3):1297-303. DOI: 10.18528/gii150025 [ Links ]
5. Ogura T, Higuchi K. Technical tips of endoscopic ultrasound-guided choledochoduodenostomy. World J Gastroenterol 2015;21(3):820-8. DOI: 10.3748/wjg.v21.i3.820 [ Links ]
6. Giovannini M, Moutardier V, Pesenti C, et al. Endoscopic ultrasound-guided bilioduodenal anastomosis: A new technique for biliary drainage. Endoscopy 2001;33(10):898-900. DOI: 10.1055/s-2001-17324 [ Links ]
7. Khashab MA, Valeshabad AK, Afghani E, et al. A comparative evaluation of EUS-guided biliary drainage and percutaneous drainage in patients with distal malignant biliary obstruction and failed ERCP. Dig Dis Sci 2015;60(2):557-65. DOI: 10.1007/s10620-014-3300-6 [ Links ]
8. Wiersema MJ, Sandusky D, Carr R, et al. Endosonography-guided cholangiopancreatography. Gastrointest Endosc 1996;43(2 Pt 1):102-6. DOI: 10.1016/S0016-5107(06)80108-2 [ Links ]
9. Wang K, Zhu J, Xing L, et al. Assessment of efficacy and safety of EUS-guided biliary drainage: A systematic review. Gastrointest Endosc 2016;83(6):1218-27. DOI: 10.1016/j.gie.2015.10.033 [ Links ]
10. Mallery S, Matlock J, Freeman ML. EUS-guided rendezvous drainage of obstructed biliary and pancreatic ducts: Report of 6 cases. Gastrointest Endosc 2004;59(1):100-7. DOI: 10.1016/S0016-5107(03)02300-9 [ Links ]
11. Komaki T, Kitano M, Sakamoto H, et al. Endoscopic ultrasonography-guided biliary drainage: Evaluation of a choledochoduodenostomy technique. Pancreatology 2011;11(Suppl 2):47-51. DOI: 10.1159/000323508 [ Links ]
12. Maranki J, Hernández AJ, Arslan B, et al. Interventional endoscopic ultrasound-guided cholangiography: Long-term experience of an emerging alternative to percutaneous transhepatic cholangiography. Endoscopy 2009;41(6):532-8 DOI: 10.1055/s-0029-1214712 [ Links ]
13. Maluf-Filho F, Retes FA, Neves CZ, et al. Transduodenal endosonography-guided biliary drainage and duodenal stenting for palliation of malignant obstructive jaundice and duodenal obstruction. JOP 2012;13(2):210-4. [ Links ]
14. Vila JJ, Pérez-Miranda M, Vázquez-Sequeiros E, et al. Initial experience with EUS-guided cholangiopancreatography for biliary and pancreatic duct drainage: A Spanish national survey. Gastrointest Endosc 2012;76(6): [ Links ]
15. Kawakubo K, Isayama H, Kato H, et al. Multicenter retrospective study of endoscopic ultrasound-guided biliary drainage for malignant biliary obstruction in Japan. J Hepatobiliary Pancreat Sci 2014;21(5):328-34. DOI: 10.1002/jhbp.27 [ Links ]
16. Park DH, Lee SS, Lee TH, et al. Anchoring flap versus flared end, fully covered self-expandable metal stents to prevent migration in patients with benign biliary strictures: A multicenter, prospective, comparative pilot study (with videos). Gastrointest Endosc 2011;73(1):64-70. DOI: 10.1016/j.gie.2010.09.039 [ Links ]
17. Rai P, Singh A, Rao RN, et al. First report of endoscopic ultrasound-guided cholecystogastrostomy with a Nagi covered metal stent for palliation of jaundice in extrahepatic biliary obstruction. Endoscopy 2014;46(Suppl 1)UCTN:E334-5. DOI: 10.1055/s-0034-1377286 [ Links ]
18. Moon JH, Choi HJ, Kim DC, et al. A newly designed fully covered metal stent for lumen apposition in EUS-guided drainage and access: A feasibility study (with videos). Gastrointest Endosc 2014;79(6):990-5. [ Links ]
Received: May 16, 2017; Accepted: November 06, 2018











 texto em
texto em