Meu SciELO
Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Farmacia Hospitalaria
versão On-line ISSN 2171-8695versão impressa ISSN 1130-6343
Farm Hosp. vol.37 no.2 Toledo Mar./Abr. 2013
https://dx.doi.org/10.7399/FH.2013.37.2.117
ORIGINALES
Extemporaneous clobazam suspensions for paediatric use prepared from commercially available tablets and pure drug
Suspensiones extratemporáneas de clobazam para uso pediátrico preparadas a partir de tabletas disponibles comercialmente y fármaco puro
F. Buontempoa,b,*, M. A. Morettona,c,*, E. Quirogad, D. A. Chiappettaa,c
aDepartment of Pharmaceutical Technology, Faculty of Pharmacy and Biochemistry, University of Buenos Aires, Argentina.
bPharmacy Service, Pediatric Hospital J.P. Garrahan.
cNational Science and Technical Research Council (CONICET).
dQuality Control Laboratory, College of Pharmacists of Buenos Aires Province, Argentina.
*Authors equally contributed to this work.
The authors acknowledge the financial support for this work provided by UBACyT 2011-2014 (Grant 20020100300088).
ABSTRACT
Objective: Two clobazam aqueous suspensions for paediatric oral usage (5 mg/ml) were investigated to determinate its physicochemical stability under different storage conditions.
Method: Formulations were stored at 4 and 25 oC and the clobazam content was determined by High Performance Liquid Chromatography. Each sample was analyzed by triplicate at different time points (0, 7, 14, 28 and 56 days).
Results: Liquid suspensions were successfully formulated from pure drug and commercially available tablets. In both cases, samples showed suitable physical stability. Clobazam was chemically stable in aqueous suspension during the 56 days of the study at the two storage temperatures.
Conclusions: All the tried oral liquid formulations can be conserved at 4 and 25 oC at least 56-day period.
Key words: Antiepileptic drugs; Clobazam; Epilepsy; Paediatric oral suspension; Stability.
RESUMEN
Objetivo: Dos suspensiones orales acuosas de clobazam para uso pediátrico (5 mg/ml) fueron evaluadas para determinar su estabilidad fisicoquimica bajo diferentes condiciones de almacenamiento.
Métodos: Las formulaciones fueron conservadas a 4 y 25 oC y el contenido de clobazam fue determinado mediante Cromatografía Líquida de Alta Performance. Cada una de las muestras fue analizada por triplicado a diferentes tiempos (0, 7, 14, 28 y 56 días).
Resultados: Las suspensiones fueron formuladas satisfactoriamente a partir del principio activo puro y de comprimidos disponibles comercialmente. En ambos casos, las muestras presentaron una adecuada estabilidad física. El clobazam fue químicamente estable en las suspensiones acuosas durante los 56 días de duración del estudio a las dos temperaturas elegidas para su conservación.
Conclusiones: Todas las formulaciones orales líquidas formuladas y evaluadas en este estudio pueden ser conservadas a 4 y 25 oC por al menos 56 días.
Palabras clave: Drogas antiepilépticas; Clobazam; Epilepsia; Suspensión oral pediátrica; Estabilidad.
Introduction
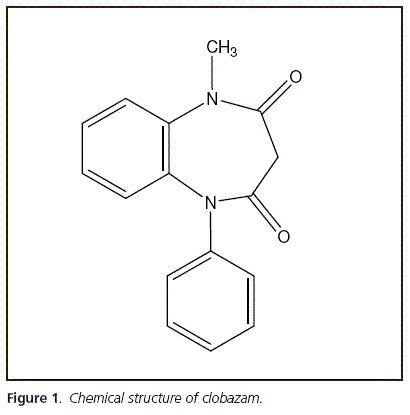
Epilepsy is a chronic neurological disorder which affects between 0.5% and 1.0% of paediatric population1. It involves recurrent unprovoked seizures and it is the third most frequent neurological disorder, after cerebrovascular disease and dementia2. Typical pharmacological treatment includes antiepileptic drugs (AEDs) that control seizures when they are daily administered3. Unfortunately one fifth of children develop Intractable Childhood Epilepsy4 where seizures became resistant to pharmacological control. Between the AEDs administered, clobazam (CLB) is a 1,5-benzodiazepine (figure 1) which presents anticonvulsant pharmacological properties5 widely used for adjunctive therapy on epilepsy treatment6. Actually, it is approved for clinical use as an AED in more than one hundred countries1. Also it has been recently approved by The US Food and Drug Administration as add-on therapy for seizures associated with Lennox-Gastaut syndrome7. CLB has been effective against a broad spectrum of seizure types in most patients for both short-term and long-term treatment8. Even more, CLB resulted useful in refractory seizures control9. Due to its unique chemical structure, in comparison with the traditional 1,4-benzodiazepines, a reduction on its anxiolytic properties and collateral effects is observed1. Its major metabolite in humans is N-desmethylCLB which is pharmacologically active5. CLB is excreted unchanged and as metabolites mainly in the urine.
CLB is a white or almost white crystalline powder which is slightly soluble in water10. It is well absorbed after oral ingestion and it presents a high oral bioavailability. Usually, oral dose recommended for children, between 1 month and 12 years, is between 0.5 and 1.0 mg/kg/day11. It is currently available as 10 and 20 mg tablets but unfortunately, no suitable liquid formulation for paediatric administration is commercially available12.
The lack of paediatric liquid dosage forms represents a challenge for hospital and community pharmacists. Usually, children require smaller doses than adults which are adjusted by body weight. Thereby solid dosage forms must be fractionated in order to fit paediatric dosages. This practice represents a concern issue because correct dosing must be ensured13. Preparing extemporaneous liquid formulations using tablets is one of the most common practices employed to adjust doses for paediatric patients. More over, children under 7 years old are unable to swallow capsules or tablets. Liquid formulations which are flavored aqueous solutions, syrups, or suspensions, are administered directly into the child's mouth by drop, spoon, or oral dispenser or incorporated into the child's food14. General dose volumes are < 5 ml for children under 5 years and < 10 ml for those of 5 years and over15.
Among paediatric pharmacy practice, traditional compounding techniques have become a useful tool in order to develop not commercially available liquid dosage forms16,17. In this context, liquid formulations facilitate oral administration and enhance children treatment adherence. An appropriate design of a liquid dosage form requires not only a comprehensive analysis of chemical and physical considerations of drugs and pharmaceutical excipients but also evaluation of drug stability in the mid-long term as well as drug effectiveness, tolerance and formulation safety16.
The purpose of this study was to prepare an optimal paediatric extemporaneous oral liquid CLB suspension from commercially available tablets and pure drug to determine the physical and chemical stability of these products for up to 56 days, stored at 4 and 25 oC. In this way, these formulations allowed to study the compounding techniques versatility on developing liquid CLB dosage forms starting from different sources. In addition to the best of our knowledge, no studies have been published describing physical and chemical stability of an oral CLB suspension.
Materials and methods
Materials
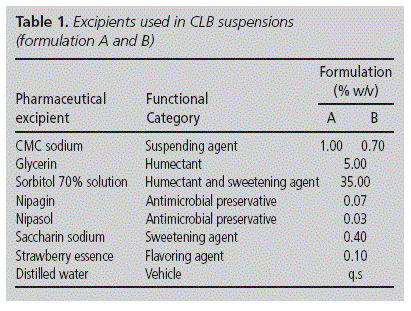
Clobazam tablets (Sanofi - Aventis, batch: 707173) were obtained from Hospital pharmacy (Paediatric Hospital J.P. Garrahan, Buenos Aires, Argentina) and clobazam (Parafarm batch: E9A001) was purchased from Saporitti (Buenos Aires, Argentina). Methylparaben (Nipagin) (D. Prest, batch: 200B032S), propylparaben (Nipasol) (D.Chutrau, batch: LI1B14), saccharin sodium (Stanton, batch: 40704), strawberry essence (D. Prest, batch B410), glycerin (D. Prest, batch: 090S0S), sorbitol solution, 70% (Van Rossum SRL, batch 808181760), carboxymethylcellulose sodium (CMC sodium) of high viscosity (Parafarm, batch: 508081) and solvents of HPLC grade were used as received.
Formulations preparation
In the present study we prepared two CLB suspensions denominated Formulation A and B:
- CLB (pure drug) 0.5 %w/v and CMC sodium 1 %w/v (Formulation A).
- CLB commercial tablets (equivalent to 0.5 %w/v of CLB) and CMC sodium 0.7 %w/v (Formulation B).
The rest of pharmaceutical excipients for each formulation are detailed in table 1. The suspensions were prepared as follows:
Formulation A
First, nipagin and nipasol were solubilized in ¼ total volume of distilled water at 90 oC. Once the preservative agents were dissolved, the solution was cooled and saccharin sodium was added. Separately, CLB (pure drug) was placed into a mortar and triturated with CMC sodium. Next a right amount of glycerin, containing the strawberry essence, was added in a constant shaking until a homogeneous smooth paste is formed. After the glycerin incorporation is completed, CLB was completely dispersed. In the next place, sorbitol 70% and the preservative agent solutions were both incorporated to the previous suspension and homogenized.
Finally, the suspension volume was brought to 100 ml with distilled water. Later CLB suspension was shaken until homogeneity. The formulations were conserved in amber glass vials under refrigeration (4 oC) and controlled room temperature (25 oC), in the absence of light. Before removing the samples, containers were agitated to ensure uniform redispersion of the content. At least three batch of every formulation were prepared.
Formulation B
The same technique was followed as Formulation A. In this case, CLB tablets were grinded to fine powder in a mortar before use.
The elaborated suspensions were physical and chemical characterized as we describe below.
HPLC assay
Clobazam content of formulations were tested by High Performance Liquid Chromatography (HPLC). The liquid chromatographic system consisted of an isocratic solvent delivery pump (Shimadzu LC-20AT) which pumped a mixture of 50% acetonitrile and 50% water through a 125 mm x 4 mm reversed-phase C-18, 5-um column (LiChrospher® 100 RP-18, LiChroCART® 125-4) at 1.0 ml/min. The column was maintained at room temperature. The samples (20 μl) were introduced into the liquid chromatographic system using an injector (Rheodyne 7725i). The column effluent was monitored with a variable wavelength ultraviolet detector (Waters 486) at 290 nm. The analytical method was carried out according to the specifications in British Pharmacopoeia 98. Stock CLB solutions were prepared by dissolving the drug in methanol (50 μg/ml). Linearity was evaluated by analyzing a set of CLB solutions ranging from 10 to 400 μg/ml (R2 0.9998). The specificity of the analytical method in the presence of pharmaceutical additives and CLB degradation products was evaluated from CLB stock solution (50 μg/ml) and pharmaceutical additives solution without CLB. Both were subjected to forced acidic degradation (1N HCl), basic degradation (1N NaOH) and oxidation (10% H2O2) at 100 oC for 45 minutes. Aliquots were neutralized and filtered (0.45 m cellulose nitrate membranes, Whatman® GmbH, Germany) before being analyzed.
pH determination
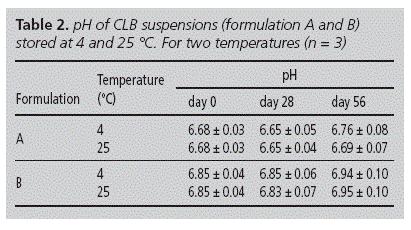
The apparent pH was determined at 0, 28 and 56 days. Examination was conducted with a digital pH-meter IQ 140 (IQ Scientific Instruments, CA, USA). Each measurement was done by triplicate and then the results were averaged.
Sedimentation volume
A suitable parameter, for assessing the physical stability of a suspension, is the sedimentation volume which is defined as:
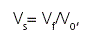
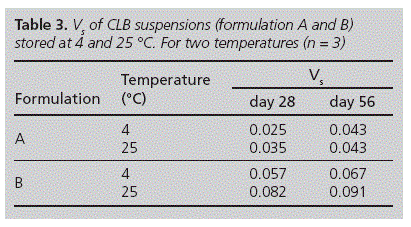
Where Vs is the volume ratio, Vf and Vo are the final settled volume and the original suspension volume, respectively. In the present study, samples (50 ml) were hand shaked for 30 seconds in order to asses the content uniformity and transferred from amber glass vials to a 50 ml-graduated cylinder protected from light and sealed with a rubber cap. Then, Vs was determined after samples were allowed to stand for 56 days at 4 and 25 oC. Assays were done by triplicate and then the results were averaged.
Viscosity determination
Viscosity, which is the inverse of fluidity, could vary after storage at different conditions. Thereby, samples (150 ml) dynamic viscosity was tested with a Brookfield Rotational Viscometer RVT-Spindle #2 (Massachusetts, USA) at 1, 2.5, 5, 10, 20 and 50 RPM, with time intervals of 60 seconds, at day 0, 28 and 56. Viscosity was measured three times in units of milliPascal x seconds (mPa.s) and the results were averaged.
Stability study
In order to evaluate CLB chemical stability under different storage conditions, CLB suspensions were storage at 4 and 25 oC in amber, glass vials (30 ml) in the absence of light. Then, aliquots were collected from each container on days 0, 3, 7, 14, 28 and 56. Samples were properly diluted (50 μg/ml) with HPLC mobile phase in order to fit the calibration range curve and immediately analyzed by HPLC. CLB stability in each liquid formulation was determined by calculating the percentage of the initial CLB concentration remaining at each time point. Stability was defined as the retention of not less than 90% of the initial concentration.
Morphology
The morphological analysis of CLB suspended particles was conducted by means of optical microscopy (Trinocular Microscope Arcano XSZ-107 E) with photographic digital camera. The photos were analyzed using TSView Version 6.2.4.5 for Windows.
Results
Preliminary studies investigated the development of CLB solution with a high concentration of cosolvent such as propyleneglycol (60 %v/v) and sorbitol 70% solution (30 %v/v), in order to enhance CLB aqueous solubility. However, CLB extemporaneous solution presented low physical stability and drug remaining in solution only for 7 days. Also, formulations with lower concentration of propyleneglycol presented drug precipitation at 48 h.
Therefore, an aqueous suspension results a useful dosage form for administering a water-poorly soluble or aqueouschemical unstable drug. This is the main reason why suspension development appears as an excellent approach for CLB liquid formulation containing a quantity of drug in an acceptable volume13,18-21. Also, suspensions could mask CLB unpleasant taste improving paediatric formulation acceptance and treatment adherence.
The figure 2 shows the chromatograms corresponding to the suspension without CLB (A), CLB standard solution (50 μg/ml) (B), CLB subjected to acid degradation (C), CLB subjected to alkaline degradation (D) and CLB subjected to oxidation (E). The CLB retention time was 3.48 min. The relative error and the CV (coefficient of variation) were lower than 10%. It was shown that the analytical method was able to separate the CLB peak of the degradations peaks and there was no interference between CLB standard, degradation products and suspension control retention times.
To assess the CLB stability in paedriatic oral formulations, extemporaneous oral liquid CLB suspensions from commercially available tablets and pure drug were assayed. These suspensions were prepared with excipients commonly used in paediatric formulations22 and they were stored at two different temperatures (4 and 25 oC) for 56 days. In both cases, suspensions were easily re-dispersed after simple handshaking (30 seconds) and pH values did not show an important variation along the assay (table 2). However, the Vs increased between 0 and 56 days, for both storage temperatures (table 3). Notice that formulations stored at 4 oC showed lower Vs values than their counterparts stored at 25 oC for 28 and 56 days.
Figure 3 shows the dynamic viscosity as a function of the rotational speed, for samples A and B. Both, the upward and downward curves, presented identical pathways which suggested a pseudoplastic flow without thixotropy. Also, a difference could be observed between formulation A and B viscosity values. Formulation B showed a higher viscosity values than formulation A.
Formulation A showed an increase on viscosity values after 28 and 56 days under refrigerated conditions (4 oC). However, the samples viscosity was reduced when the storage was perform at 25 oC (table 4). A similar trend was followed by formulation B. Nevertheless, the viscosity variation at 4 oC was less marked and values remain almost unchanged.
According to the chemical characterization, CLB stability was followed by HPLC. Table 5 shows the initial CLB concentration remaining percentage up to 56 days. Encouraging results were obtained for both formulations as to CLB chemical stability. Formulations (A and B) presented a CLB remaining percentage above 98% for both storage conditions (4 and 25 oC).
The morphological characterization of the suspended CLB particles is showed on figure 4. Notice the presence of irregular CLB particles and the higher amount of suspended particles in formulation B (figure 4B).
Discussion
The aim of the present study was to develop an optimal oral liquid CLB suspension in order to enhance physical-chemical stability of the drug and paediatric patient acceptance.
As it is well known, suspensions are thermodynamic unstable systems. Therefore, suspended particles would sediment at a certain sedimentation velocity. That is the main reason why they have to be shaken before use, to ensure content uniformity19. According with the Stocks Law23, changes on viscosity values could modify sedimentation velocity of the suspended particles. Generally, suspending vehicles are used to maintain dispersed particles homogeneously distributed in the formulation by keeping an appropriate viscosity. Our formulations presented a pseudoplastic flow because the hydrocolloid used as suspending agent was CMC sodium which imparts non-Newtonian properties to the suspension. This is very important because a pseudoplastic dispersion media could support two processes: 1) retard the sedimentation of small particles, as their apparent viscosities increase under the small stresses associated with sedimentation, and 2) the medium could undergo structural breakdown under the higher stresses involved in shaking and pouring24.
Although the suspending vehicule content was reduced from 1 to 0.7 %w/v. Formulation B showed a higher viscosity values than A, probably due to the amount of pharmaceutical excipients presented on commercial tablets.
As to our results, refrigerated storage conditions (4 oC) produced a slight increase on formulation viscosity leading to a decrement on sedimentation velocity of the suspended particles. Cancela et al25 observed that the viscosity of suspensions containing CMC sodium decreases when the temperature increases. This behaviour agrees with our results. Therefore, Vs values at 4 oC resulted smaller than those for samples storage at room controlled temperature for a period of 56 days.
One of the main objectives of the present work was to investigate CLB chemical stability under different storage conditions. Both kinds of formulations (A and B) demonstrated an acceptable drug chemical stability where CLB content remain above 98% at room controlled temperature and refrigerator temperature for a period of 56 days.
As to the microscopic aspect of the samples, those prepared from commercially available tablets present a higher amount of suspended particles due to the presence of water-insoluble pharmaceutical additives in the CLB tablets. Nevertheless, the same morphology of CLB particles was observed for both kinds of formulations (figure 4).
In conclusion, paediatric oral liquid CLB suspensions presented an adequate physical stability in order to keep the CLB particles homogeneously distributed in the formulation. The adequate chemical CLB stability under refrigerated and room controlled temperature conditions are of real importance, since CLB suspensions could easily be distributed and storage at a paediatric hospital without any special storage conditions.
References
1. Chu-Shore CJ, Thiele EA. New Drugs for Pediatric Epilepsy. Semin Pediatr Neurol. 2010;17:214-23. [ Links ]
2. Wasowski C, Gavernet L, Barrios IA, Villalba ML, Pastore V, Samaja G, et al. N,N-Dicyclohexylsulfamide and N,N-diphenethylsulfamide are anticonvulsant sulfamides with affinity for the benzodiazepine binding site of the GABAA receptor and anxiolytic activity in mice. Biochem Pharmacol. 2012;83:253-9. [ Links ]
3. Löscher W. The pharmacokinetics of antiepileptic drugs in rats: consequences for maintaining effective drug levels during prolonged drug administration in rat models of epilepsy. Epilepsia. 2007;48: 1245-58. [ Links ]
4. Kossoff EH. Intractable childhood epilepsy: choosing between the treatments. Semin Pediatr Neurol. 2011;18:145-9. [ Links ]
5. Koeppen D, Baruzzi A, Capozza M, Chauvel P, Courjon J, Favel P, et al. Clobazam in therapy-resistant patients with partial epilepsy: a double-blind placebo-controlled crossover study. Epilepsia. 1987; 28:495-506. [ Links ]
6. Rouini MR, Ardakani YH, Shohrati M, Hakemi L, Badri G, Mokhberi M. Pharmacokinetics and bioequivalence study of clobazam 10 mg tablet. Int J Pharmacol. 2006;5:481-4. [ Links ]
7. FDA NEWS RELEASE. FDA approves Onfi to treat severe type of seizures (citado 24/10/2011). Disponible en: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm276932.htm [ Links ]
8. Remy C. Clobazam in the treatment of epilepsy: a review of the literature. Epilepsia. 1994;35:S88-91. [ Links ]
9. Bergey GK. Evidence-based Treatment of Idiopathic Generalized Epilepsies with New Antiepileptic Drugs. Epilepsia. 2005;46:161-8. [ Links ]
10. Martindale Thirty-first Edition. Royal Pharmaceutical Society of Great Britain. London, England; 1996. [ Links ]
11. Ng Y-t, Collins SD. Clobazam. Neurotherapeutics. 2007;4:138-44. [ Links ]
12. Nahata MC, Allen LV. Extemporaneous drug formulations. Clinical Therapeutics. 2008;30;2112-9. [ Links ]
13. Juárez Olguín H, Flores Pérez C, Ramírez Mendiola B, Coria Jiménez R, Sandoval Ramírez E, Flores Pérez J. Extemporaneous suspension of propafenone: attending lack of pediatric formulations in Mexico. Pediatr Cardiol. 2008;29:1077-81. [ Links ]
14. Allen LV. Dosage form design and development. Clinical Therapeutics. 2008;30:2102-11. [ Links ]
15. Walsh J, Bickmann D, Breitkreutz J, Chariot-Goulet M; European Paediatric Formulation Initiative (EuPFI). Delivery devices for the administration of paediatric formulations: Overview of current practice, challenges and recent developments. Int J Pharm. 2011;415:221-31. [ Links ]
16. Glass BD, Haywood A. Stability considerations in liquid dosage forms extemporaneously prepared from commercially available products. J Pharm Pharm Sci. 2006;9:398-426. [ Links ]
17. Brion F, Nunn AJ, Rieutord A. Extemporaneous (magistral) preparation of oral medicines for children in European hospitals. Acta Paediatr. 2003;92:486-90. [ Links ]
18. Santoveña A, Hernández-Paiz Z, Fariña JB. Design of a pediatric oral formulation with a low proportion of hydrochlorothiazide. Int J Pharm. 2012;423:360-4. [ Links ]
19. Buontempo F, Bernabeu E, Glisoni RJ, Quiroga E, Bregni C, Chiappetta DA. Carvedilol stability in paediatric oral liquid formulations. Farm Hosp. 2010;34:293-7. [ Links ]
20. Popa L, Ghica MV. Ibuprofen pediatric suspension designed and optimized by response surface methodology. Farmacia. 2011;59:500-6. [ Links ]
21. Sosnowska K. Winnicka K, Czajkowska-Ko nik A. Stability of extemporaneous enalapril maleate suspensions for pediatric use prepared from commercially available tablets. Acta Poloniae Pharmaceutica - Drug Research. 2009;66:321-6. [ Links ]
22. Fabiano V, Mameli C, Zuccotti GV. Paediatric pharmacology: Remember the excipients. Pharmacol Research. 2011;63:362-5. [ Links ]
23. Hennart SLA, Wildeboer WJ, van Hee P, Meesters GMH. Stability of particle suspensions after fine grinding. Powder Technology. 2010; 199:226-31. [ Links ]
24. Aulton ME, Taylor K, editores. Pharmaceutics: The science of dosage form desing. 2o Edition, Churchill, Livingstone, Elsevier; 2002. [ Links ]
25. Cancela MA, Álvarez E, Maceiras R. Effects of temperature and concentration on carboxymethylcellulose with sucrose rheology. J Food Engineering. 2005;71:419-24. [ Links ]
![]() Correspondence:
Correspondence:
Correo electrónico: diegochiappetta@yahoo.com.ar
(D. A. Chiappetta)
Recibido: 7 de octubre de 2012.
Aceptado: 20 de enero de 2013.