Meu SciELO
Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Enfermería Global
versão On-line ISSN 1695-6141
Enferm. glob. vol.21 no.67 Murcia Jul. 2022 Epub 19-Set-2022
https://dx.doi.org/10.6018/eglobal.511881
Originals
Knowledge of nurses in the administration and regulation of high alert medications in oncology
1University Center Santa Cruz de Curitiba - UNISANTACRUZ, Curitiba – PR, Brazil
2Complex of the Clinical Hospital of the Federal University of Paraná, Curitiba – PR, Brazil
Objective:
To identify the knowledge of nurses about the administration and regulation of high surveillance drugs in an oncology hospital in Brazil.
Method:
Cross-sectional research with quantitative approach carried out from September to October 2021, in five hospitalization units. The participants were 26 nurses who answered Nurses’ knowledge of high alert medications. The data were analyzed by descriptive statistics and questions with scores ≥70% represented satisfactory knowledge.
Results:
Most nurses reported not having participated in training for the administration of high surveillance medications (57.7%; n=15). The overall average knowledge was 79.9%. It was observed that the average knowledge for administration of these drugs was 80.4%, and eight questions obtained a score ≥ 70% of correct answers, especially those related to the speed of infusion, the route of administration and dilution of concentrated solutions. Regarding regulation, the average knowledge was 78.6%, and the question “If the patient can tolerate, it is indicated that potassium chloride is administered orally instead of intravenously” obtained the highest error rate (50%), followed by the item “Use “Ampoule” or “vial” for dosage measurement, instead of “mg” or “g” with 46.2% incorrect answers.
Conclusion:
Nurses’ knowledge about the use and regulation of high surveillance drugs was satisfactory, although there is a need to improve it to promote safe care in the oncological context.
Keywords: Patient Safety; Nurses; Potentially Inappropriate Medication List; Medication Errors; Oncology Service, Hospital
INTRODUCTION
The World Health Organization (WHO) conceptualizes patient safety as a structure of organized activities capable of building cultures, processes, procedures, behaviors, technologies and health environments that consistently and sustainably minimize risks, reduce the occurrence of avoidable damage and reduce the probability of errors and their impacts when they happen1. This theme is the responsibility of the different teams of professionals working in complex units and systems, in which they are constantly changing, with new technologies and growing demand for integration of care delivery2.
The high rate of incidents caused by health care, especially those arising from errors related to the use of medicines, prompted the WHO, in 2017, to launch the third global challenge of patient safety with the theme “Medication without Harm”, whose objective is to improve the planning of institutions and professionals for safe practice and reduce errors related to medications3.
Medication error is defined as any prevenTable event that can cause or lead to inappropriate use of medications or cause harm to a patient, while medication is under the control of healthcare professionals, patients, or consumers. This event may be related to professional practice, health products, procedures and systems, including prescription, verbal guidance, labeling, packaging and nomenclature of industrialized and manipulated products, dispensing, distribution, administration, education, monitoring and use4.
It is known that the use of medicines to treat and prevent diseases has increased in recent years. Moreover, most of the nurses’ care processes involve the use of medications and that event related to this practice are among the most common adverse events (AE) in health services5. AE means any incident that has resulted in damage to the patient6.
In this interim, potentially dangerous drugs, also called high alert medications (HAM), have a greater potential to cause AE when there is an error in their use7. Anticoagulants, narcotics and opiates, insulin and sedatives are more associated with the occurrence of damage such as bleeding, hypoglycemia, delirium, bradycardia, among others8. In hospital care, about 80% of prescriptions have at least one HAM, with a higher frequency for antithrombotic and insulin. Among these, only 21.7% and 3.8%, respectively, they were double-checked as a strategy to reduce errors9. Moreover, the incipience of knowledge among nurses in the use of insulin, electrolytes and chemotherapy contributes to the occurrence of errors and AE10.
These data reinforce the need for research that investigates the knowledge of nurses in the theme as a way to identify strengthened and fragile areas and serve as a subsidy to carry out educational actions with a view to contributing to the quality and safety of care, especially because of the fact that these professionals are responsible for the administration of a variety of HAMs in the hospital area and in the oncological context. Thus, the question was: What is the knowledge of nurses about the administration and regulation of high surveillance drugs?
The objective of this research was to identify the knowledge of nurses about the administration and regulation of high surveillance drugs in an oncology hospital in Brazil.
METHODS
This is a cross-sectional research with a quantitative approach conducted from September to October 2021, in five hospitalization units of an oncology hospital in Brazil. The choice for this hospital occurred because it is an important reference center and excellence in diagnosis, treatment, prevention, teaching and research in the oncology area.
The population was identified through the availability of the list of employees provided by the management of the unit and totaled 40 nurses. The inclusion criteria were: being a nurse as a member of the unit investigated during the data collection period; with a minimum weekly workload of 36 hours and has been working for at least three months in the sector. Holiday professionals, on leave, on leave or whose questionnaires were returned blank and/or with less than 50% of the questions filled out were excluded. After applying these criteria, 26 nurses agreed to participate in the research, constituting an intentional and non-probabilistic sample. No participant was excluded from the analysis.
Data were collected in the facilities of the units, in rooms previously designated by the heads of the services. The invitation and clarifications to the nursing professionals were made in the work environment, individually and/or in small groups in the morning, afternoon and evening. The researchers reported the estimated time of 10 minutes to complete the data collection instrument. Those who agreed to participate, an envelope containing two forms of the Informed Consent Form (TCLE) was delivered and the instrument called Nurses’ knowledge of high alert medications11translated, adapted and validated for use in Brazil12. Permission to use the data collection instrument was requested via e-mail.
The original instrument is self-applicable and consists of 20 items that make up parts A (Administration of medicines) and B (Regulation of medicines). In the validation process for Brazilian culture, three questions were included, two in Part A and one in Part B. Thus, the Brazilian version is composed of 23 items. All items are answered by dichotomous questions, in which the participant informs true or false for each of the statements, generating a score of arbitrary units. At each correct answer, five points are computed, resulting in a possible total score of 115 points. High scores indicate a higher level of knowledge and low scores, lower level of knowledge about HAM12. It was considered satisfactory knowledge for the questions with ≥70% of correct answers13,14.
After data collection, the information of each questionnaire was inserted, by double typing, into a spreadsheet in the Microsoft Office Excel®2016 program for further processing and analysis. Descriptive statistics were used for data analysis, with categorical variables distributed in absolute and relative frequencies and continuous variables presented by means of central trend measurement (means) and dispersion measurement (standard deviation).
To comply with Resolution 466/2012, this research was initiated after approval by the Institutional Research Ethics Committee under opinion number 4,894,297. To safeguard the anonymity and confidentiality of the participants, the completed instruments were delivered to the researchers and identified with the letter E of nurse, followed by the numeral relative to the sequence of return of the envelopes (E1,E2...E40).
RESULTS
The study included 26 nurses, with predominance of females (76.9%;n=20), and with complete post-graduation (38.5%;n=10). The mean age of the participants was 33.1 years (standard deviation of 7.9). The average performance in the profession and hospital in months was 83.4 and 29.5, respectively. Most nurses reported not having participated in training for the administration of HAM (57.7%;n=15).
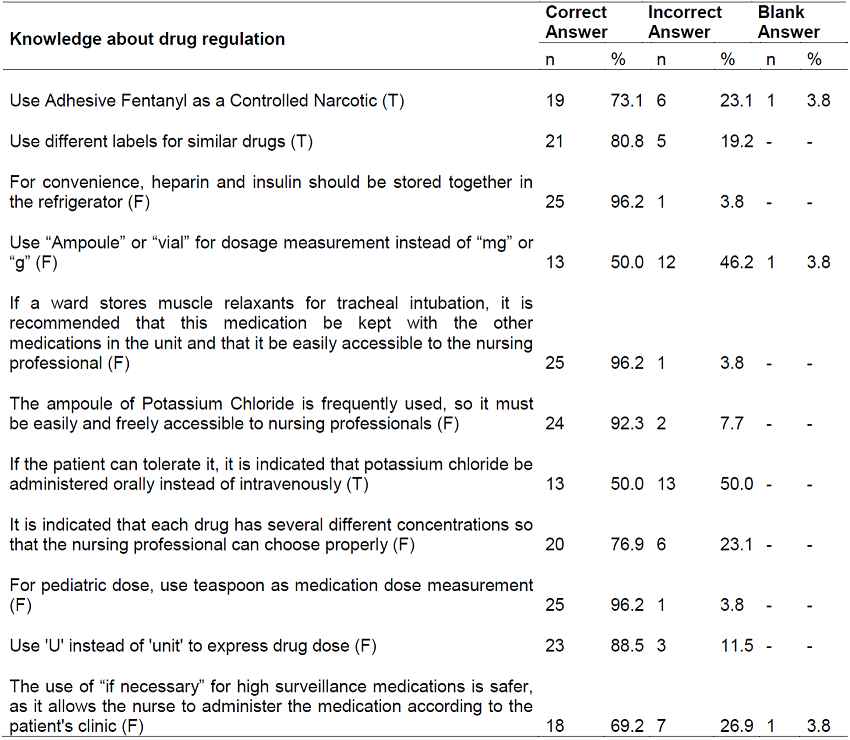
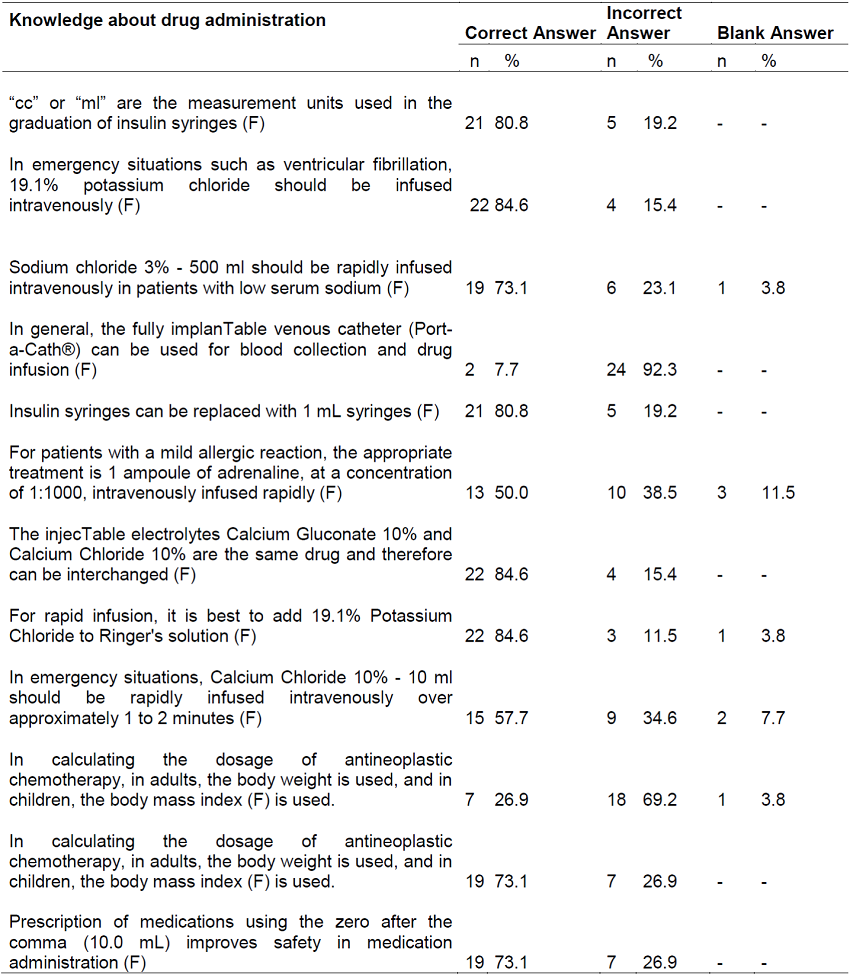
The general average knowledge of nurses for the administration and regulation of HAM was 79.4%. It was observed that the average knowledge for the administration of these drugs (Part A) was 80.4%, and eight questions in this section obtained ≥ correct response score ≥ 73% as shown in Table 1.
Table 1. Distribution of nurses' responses, according to knowledge about the administration of high-alert medications. Paraná, Brazil, 2021
Source: created by the authors.
The average knowledge for regulation of dangerous drugs was 78.6%. Six items obtained correct answer rate equal to or greater than 80%. The question “If the patient can tolerate, it is indicated that potassium chloride is administered orally instead of intravenously” obtained a correct rate similar to that of errors (Table 2).
DISCUSSION
The data show relevant aspects about the knowledge of nurses regarding the administration and regulation of HAM. It was observed that the questions that obtained correct scores higher than 96% were related to the regulation of these drugs, specifically for the questions related to the non-storage of heparin and insulin together in the refrigerator, muscle relaxers with other medications in the unit and use of a teaspoon as a measure of the dose of medication for children. Of the 2,363 nurses surveyed in 29 hospitals in Pakistan, 77.9% agreed that heparin and insulin should be stored in different locations and 74.2% disagreed with muscle relaxant storage, for example, atracurium, along with other medications and easy access to the nursing team13.
The National Patient Safety Program adopts in the safety protocol in the prescription, use and administration of medications the recommendation of the use of metric system to express the prescribed doses and warns professionals regarding the use of units of non-metric measures such as spoon, ampoule and vial7. Another relevant point is the non-use of abbreviations for the prescription of medications, as it is one of the factors that contribute to the occurrence of errors, especially the administration of insulin. The use of ‘U’ instead of ‘unit’ often corroborates for the administration of a dose ten times higher than that prescribed, because ‘U’ is confused with ‘0’, resulting in hypoglycemia, coma and death resulting from the application of high doses of insulin15.
The number of correct answers in this research regarding the use of the letter “U” to define “units” was 88.5%, different from that found among the knowledge of nursing professionals in a large hospital working in intensive care sectors in which 68% chose the correct option for this item16. On the other hand, the opposite occurred in the question “Use ampoule or vial for dosage measurement, instead of milligram or gram”, in which 46.2% of the participants answered incorrectly while research in Turkey, 94.8% of the interviewees correctly answers this statement15.
It is observed that half of the professionals investigated showed not to recognize the oral route as a form of potassium administration instead of intravenous, contrary to good safe practices for the prevention of errors involving concentrated potassium chloride, in which it is recommended, whenever possible, to re-use oral potassium for the treatment of hypokalemia17. However, 92.3% of nurses recognize that it is a restricted-use drug and should be stored judiciously, especially for injecTable solutions.
This denotes that the vast majority of nurses seem to have knowledge of the risks associated with mismanagement of potassium, and the difficulty of access to this electrolyte becomes an effective and safe measure to minimize errors, differently from that found in a study conducted in a hospital conducted in a hospital located in northeastern Brazil, which identified nurses’ incipient knowledge about correct storage, in which it represented the second item with the highest error score (46.2%)10.
It is noteworthy that concentrated electrolytes when administered intravenously predispose patients to the highest risk, leaving them more vulnerable to the occurrence of severe damage, which reinforces the importance of the nurse’s knowledge about all the stages that make up the process of the medication system adopted by the health organization, given that incorrect administration of intravenous potassium contributes to episodes of arrhythmias, cardiac arrest and deaths13.
It is noted that more than two thirds of the nurses indicated the correct option in relation to the speed and parenteral administration of potassium and sodium chloride, which differs from Palestinian research whose objective was to evaluate the knowledge of nurses in the theme. The above-mentioned research pointed out that the most common administration errors were related to the lack of knowledge about intravenous drug administration, with emphasis on the application of electrolytes in bolus14. It is worth mentioning that in relation to the rapid infusion of calcium chloride 10%, about 43% of the nurses surveyed here answered wrong or did not answer, in which it shows an unsatisfactory knowledge and an opportunity for improvement to be managed by the continuing education service, because the infusion of this electrolyte should be slowly and in a vessel in order to minimize the sensation of skin burning and peripheral vasodilation14.
Considering that errors related to the use, handling and storage of anticoagulants, hypoglycemic agents, neuromuscular blockers and concentrated electrolytes are responsible for the occurrence of AE, the identification, packaging, preparation and correct administration of these drugs, especially for concentrated/hypertonic solutions, are barriers that prevent serious and fatal injuries to the assisted patient. Thus, the restricted use, the separation and proper labeling of similar drugs, such as heparin and insulin, the use of appropriate syringes for correct administration of the prescribed dose and the incentive to double checks by nurses to prepare and administer HAMs, become protective measures to reduce errors and correlated damage14.
According to the data obtained, 76.9% of the nurses answered as false the question about the availability of different concentrations to facilitate therapeutic management, evidencing the knowledge of these professionals in important aspects in the prevention of errors. This result is contrary to the knowledge of nurses working in care units of a university hospital located in the northeast region of Brazil, in which 80.8% are unaware of this action as a protective measure to minimize errors caused by the use of HAMs10. In a cross-sectional study conducted with 280 nurses from seven hospitals in Palestine, it was pointed out that less than half of the professionals answered that this statement was correct and 7.1% could not answer, indicating fragility of knowledge14.
It is recognized that reducing the presentations of the same drug in terms of concentration and volume is an important barrier to attenuate the occurrence of AE in health institutions17. The creation of strategies with the implementation of educational, organizational actions and development of new technologies are fundamental to mitigate the occurrence of medication errors in hospital units18, especially considering that insufficient knowledge and the absence of standard operating procedure (POP) defined for the HAMs were pointed out as obstacles to safe administration16. In a study conducted in Palestine14and Pakistan19, the prevalent obstacle in relation to the administration of these drugs was the conflict of opinions between the nurse and the physician with 37.9% and 63.7%, respectively. Although the present research has not investigated the perception of nurses regarding contributing factors to incur errors associated with HAMs, these data denote the need to improve communication between professional categories to ensure excellent care and with the lowest possible risk14, including with the pharmacy team.
In the hospital under study, clinical pharmacists are responsible for validating the medical prescription for the total volume, dose of added components, therapeutic indication and among other indispensable elements for process safety such as the preparation, handling and dispensation of antineoplastic drugs for further administration and monitoring of desirable effects, or not, by care nurses. This work process, in part, justifies the nurses’ ignorance about the calculation of antineoplastic drugs in adults and children, similarly to that found in international13,15and national10research.
On the other hand, it denotes the fragility of generalist education by nursing schools, a situation aggravated by the fact that nurses are recognized by Resolution n. 220 of the Brazilian Ministry of Health as a member of the multidisciplinary team of antineoplastic therapy and co-responsible for the evaluation of the medical prescription prior to administration20. This should verify the relevance of the drugs to the prescribed protocols, release parameters informed by the doctor, weight and height of the patient and their respective body surface, dose, dilution, incompatibilities, infusion time and route of administration21, being paramount the formal qualification by continuing education services compatible with priority areas to provide safe care, with quality and based on the best scientific evidence for treatment to cancer patients.
Considering that most chemotherapy doses are determined based on the body surface area14, it is emphasized that having satisfactory knowledge regarding dose administration proportional to the physical constitution of the patient is a strong ally in promoting safe practices, as these professionals can anticipate circumstances that may compromise care security, in order to avoid cases of toxicity, severe AE and deaths involving the cytotoxic properties of antineoplastic drugs. In addition, it is important to recognize signs and symptoms of acute infusion reactions as well as management and possible treatment.
Adrenaline is an intense peripheral vasoconstrictor used in cardiac emergencies, anaphylaxis reactions and anaphylactic shock, hemorrhages, or acute exacerbation of asthma. It can be administered intramuscularly, subcutaneously and intravenously, the latter being done with reservation because it should be slowly and diluted (1:10,000 or 1:100,000), while subcutaneously in solution 1:1,00022. In the meantime, it was observed that approximately half of the nurses answered incorrectly regarding the concentration and route of adrenaline administration used in cases of mild reactions. It is relevant to improve pharmacological and care knowledge in the administration of HAMs as a way to ensure adequate care and collaborate to build the patient safety culture to promote safer care and with the lowest possible risk, and to advance the indicators of specific results in the area of safety and care quality.
The question that obtained the highest rate of incorrect answers was blood collection and infusion of drugs usually performed by the fully implanTable venous catheter (CTI) (Port-a-Cath®), which obtained 92.3% of errors, being higher than that found in a study with nurses from Turkey (64.9%)15, and in a multicentric research conducted in hospitals in Pakistan (39.4%)13. Although the collection of blood samples by these catheters is a complex issue, there is evidence of not routinely using them for this purpose, as in addition to being a significant risk factor for bloodstream infections, especially, in neutropenic patients, there is a marked risk of inaccurate results, an aggravated situation if there is omission of essential steps to obtain adequate samples by the health team, for example, failures in sterile and aseptic precautions, maximizing errors in the pre-analytical phase23,24.
It is necessary that the collection team has competence to perform the procedure, with special attention to the care that precedes the obtaining of catheter samples that were previously used for the administration of medications and solutions, as well as patients who need to perform coagulogram, since heparin is usually used in some health services to maintain the permeability of venous access. This circumstance has the potential to interfere in the laboratory result, because these samples are more likely to produce false-positive results, reinforcing there are times when peripheral samples are priority and necessary to confirm the laboratory diagnosis23.
In the hospital under study, exclusive and reference for cancer care, it has some particularities in relation to the use of Port-a-Cath® for blood sample collection. One of them is the existence of specific POP for this procedure and extensive knowledge of nurses. Another factor is the profile of patients who are seen, often with a fragile peripheral venous network and routine laboratory tests. In general, the ICU provides a facilitating access to blood collection and use of therapy, improving quality of life and reducing pain caused by recurrent and unsuccessful peripheral punctures by nurses. Moreover, as there is no consensus on the use of solutions with or without heparin to maintain the permeability of these devices in cancer patients25, salinization was adopted in the present institution in view of the benefits provided to oncological-hematologic patients.
This practice means to further reduce the exceptions for the non-removal of blood from these catheters provided that it is preceded by actions aimed at reducing the risk of obstruction and infection such as the performance of flushing in the pathway with 10 ml and 20 ml of saline solution 0.9% after the administration of any drugs and after reflux or blood collection, administration of blood products or contrast, respectively21. It is conjecture that these elements may have contributed to obtain discrepant results of this issue when compared with the knowledge of nurses identified in other national and international studies, becoming a potential confounding factor, considering the specificities of this care context and the protocols adopted by the oncologic services, and limiting the instrument of data collection itself when applied to the oncological area.
The conductive of the research in a single oncology hospital, with a relatively small sample due to the refusal of participation of about half of the target population, prevents the generalization of the data that added to the cross-sectional design that did not allow establishing cause-effect relationships and strictly descriptive analysis constitute the limitations of this investigation.
CONCLUSION
The nurses’ knowledge about the use of high surveillance drugs was satisfactory, although there is a need to improve it in relation to the administration of concentrated solutions regarding the infusion speed, dilution and route of administration, the use of abbreviations and dose expressions, the use of different labels, the storage of neuroblockers and the dose calculation of chemotherapy drugs.
In this perspective, encouraging formal education in the theme and training these professionals through continuing education, become timely and relevant strategies to improve the knowledge of nurses and promote the safety of cancer patients in the use of high surveillance medications.
REFERENCIAS
1. World Health Organization. Global patient safety action plan 2021-2030: towards eliminating avoidable harm in health care [Internet]. Geneva: WHO; 2021 [acesso em 10 fev. 2022]. Disponível em: https://www.who.int/teams/integrated-health-services/patient-safety/policy/global-patient-safety-action-plan [ Links ]
2. Lachman P. New challenges for patient safety. Int. j. qual. health care [Internet]. 2019 [acesso em 22 out. 2021];31(2):73-74. Disponível em: https://doi.org/10.1093/intqhc/mzz010 [ Links ]
3. Word Health Organization. Who launches global effort to halve medication-related errors in 5 years [Internet] Geneva: WHO; 2017 [acesso em 24 nov. 2021]. Disponível em: https://www.who.int/news/item/29-03-2017-who-launches-global-effort-to-halve-medication-related-errors-in-5-years [ Links ]
4. Brasil. Ministério da Saúde. Resolução de Diretoria Colegiada nº 406, de 22 de julho de 2020 [Internet]. Diário Oficial da União; 2020 [acesso em 21 jan. 2022]. Disponível em: https://www.in.gov.br/en/web/dou/-/resolucao-de-diretoria-colegiada-rdc-n-406-de-22-de-julho-de-2020-269155491 [ Links ]
5. Conselho Regional de Enfermagem do Estado de São Paulo. Uso seguro de medicamentos. Guia para preparo administração e monitoramento [Internet]. São Paulo: COREN-SP; 2017 [acesso em 22 nov. 2021]. Disponível em: https://portal.coren-sp.gov.br/sites/default/files/uso-seguro-medicamentos.pdf [ Links ]
6. Word Health Organization. Conceptual framework for the international classification for patient safety. Version 1.1. Final Technical Report. [Internet]. Genova: WHO; 2009. [acesso em 12 jul. 2021]. Disponível em: https://www.who.int/patientsafety/taxonomy/icps_full_report.pdf [ Links ]
7. Brasil. Ministério da Saúde. Protocolo de Segurança na Prescrição, uso e Administração de Medicamentos [Internet]. Brasília; 2013 [acesso em 14 set. 2021]. Disponível em: https://portaldeboaspraticas.iff.fiocruz.br/biblioteca/protocolo-de-seguranca-na-prescricao-uso-e-administracao-de-medicamentos/ [ Links ]
8. Institute for Healthcare Improvement. High-Alert Medication Safety [Internet]. Massachusetts, EUA; 2021 [acesso em 19 fev. 2022]. Disponível em: http://www.ihi.org/Topics/HighAlertMedicationSafety/Pages/default.aspx [ Links ]
9. Arduini GO, Cordeiro ALPC, Engel RH, Stacciarini TSG, Rezende MP, Ferreira LA. Medicamentos de alta vigilância: frequência e dupla checagem em um hospital de ensino. Rev. enferm. atenção saúde [Internet]. 2018 [acesso em 19 mai. 2021];7(3):14-26. Disponível em: https://pesquisa.bvsalud.org/portal/resource/fr/biblio-970378 [ Links ]
10. Santos GO, Farre AGMC, Santana ITS, Rocha HMN, Carvalho AA, Santos GKBB, et al. Knowledge about the use of potentially dangerous drugs among hospital health care nurses. Rev Rene [Internet]. 2020 [acesso em 12 ago. 2021];21:e444662020. Disponível em: http://periodicos.ufc.br/rene/article/view/44466 [ Links ]
11. Hsaio GY, Chen IJ, Yu S, Wei IL, Fang YY, Tang FI. Nurses' knowledge of high-alert medications: instrument development and validation. J Adv Nurs [Internet]. 2010 [acesso em 12 abr. 2021];66(1):177-90. Disponível em: https://doi.org/10.1111/j.1365-2648.2009.05164.x [ Links ]
12. Apolinário PP, Rodrigues RCM, Silva JB, Secoli SR, Lima MHM. Tradução, adaptação e praticabilidade do Nurses' knowledge of high alert medications para a cultura brasileira. Rev. Eletr. Enf [Internet]. 2015 [acesso em 12 abr. 2021];17(3):1-11. Disponível em: https://revistas.ufg.br/fen/article/view/30299/20735 [ Links ]
13. Salman M, Mustafa ZU, Rao AZ, Khan Q, Asif N, Hussain K, et al. Serious Inadequacies in High Alert Medication-Related Knowledge Among Pakistani Nurses: Findings of a Large, Multicenter, Cross-sectional Survey. Front. Pharmacol [Internet]. 2020 [acesso em 25 out. 2021]; 11:1-7. Disponível em: https://doi.org/10.3389/fphar.2020.01026 [ Links ]
14. Zyoud SH, Khaled SM, Kawasmi BM, Habeba AM, Hamadneh AT, Anabosi HH, et al. Knowledge about the administration and regulation of high alert medications among nurses in Palestine: a cross-sectional study. BMC Nurs [Internet]. 2019 [acesso em 19 set. 2021]; 18 (11): 1-17. Disponível em: https://doi.org/10.1186/s12912-019-0336-0 [ Links ]
15. Günes Ü, Öztürk H, Efteli E. Nurses' Knowledge Level about High-Alert Medications. MAKU J. Health Sci. Inst [Internet]. 2021 [acesso em 12 jan. 2022]; 9(1):12-20. Disponível em: https://doi.org/10.24998/maeusabed.803284 [ Links ]
16. Pereira LN, Caon S, Pinto NA, Maciel FH, Sempé TS, Pizzol TSD. Nurses' knowledge of high-alert medications in a large-size university hospital. Rev Bras Farm Hosp Serv Saude [Internet]. 2021 [acesso em 4 out. 2021];12(2):567. https://doi.org/10.30968/rbfhss.2021.122.0567 [ Links ]
17. Instituto para Práticas Seguras no Uso de Medicamentos. ISMP Brasil emite alerta de segurança sobre o risco de troca de frascos de cloreto de potássio injetável [Internet]. Belo Horizonte: MG; 2016 [acesso em 10 fev. 2022]. Disponível em: http://www.ismp-brasil.org/site/noticia/ismp-brasil-emite-alerta-de-seguranca-sobre-o-risco-de-troca-de-frascos-de-cloreto-de-potassio-injetavel/ [ Links ]
18. Mieiro DB, Oliveira EBC, Fonseca REP, Mininel VA, Zem-Mascarenhas SH, Machado RC. Strategies to minimize medication errors in emergency units: an integrative review. Rev. Bras. Enferm [Internet]. 2019 [acesso em 3 jul. 2021];72:(suppl 1):307-14. Disponível em: https://doi.org/10.1590/0034-7167-2017-0658 [ Links ]
19. Younis I, Shaheen N, Bano S. Knowledge & practice about administration of high alert medication in the tertiary care hospital in lahore. International Journal of Health, Medicine and Nursing Practice [Internet]. 2021 [acesso em 22 out. 2021];3(4):1-16. Disponível em: https://doi.org/10.47941/ijhmnp.644 [ Links ]
20. Brasil. Ministério da Saúde. Agência Nacional de Vigilância Sanitária. Resolução nº 220, 21 de setembro de 2004 [Internet]. Diário Oficial da União; 2004 [acesso em 19 fev. 2022]. Disponível em: https://bvsms.saude.gov.br/bvs/saudelegis/anvisa/2004/rdc0220_21_09_2004.html [ Links ]
21. Moura VT. Manual de Oncologia Clínica do Brasil - Enfermagem. São Paulo: Dendrix Edição e Design Ltda, 2020. [ Links ]
22. Hipolabor Farmacêutica Ltda. Adren (Epinefrina) [Internet]. Belo Horizonte: MG; 2016 [acesso em 29 out. 2021]. Disponível em: http://200.199.142.163:8002/FOTOS_TRATADAS_SITE_14-03-2016/bulas/30621.pdf [ Links ]
23. Gorski L, Hadaway L, Hagle ME, McGoldrick M, Orr M, Doellman D. Infusion therapy standards of practice. J Infus Nurs [Internet]. 2016 [acesso em 29 out. 2021];39(1S):160. Disponível em: https://source.yiboshi.com/20170417/1492425631944540325.pdf [ Links ]
24. Chopra V. Central venous access devices and approach to device and site selection in adults. UpToDate [Internet]. Waltham (MA): UpToDate; 2021 [acesso em 17 jan. 2022]. Disponível em: https://www.uptodate.com/contents/central-venous-access-devices-and-approach-to-device-and-site-selection-in-adults/print#H1704678845 [ Links ]
25. Caponi IM, Pacheco PQC, Silva LR, Souza SR. Estrategias de prevención de la obstrucción en catéteres centrales totalmente implantados en pacientes oncológicos. Enferm. Glob [Internet]. 2020 [acesso em 19 fev. 2022];19(60):483-524. Disponível em: https://dx.doi.org/10.6018/eglobal.414531 [ Links ]
Received: February 19, 2022; Accepted: April 02, 2022











 texto em
texto em