Meu SciELO
Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Medicina Oral, Patología Oral y Cirugía Bucal (Internet)
versão On-line ISSN 1698-6946
Med. oral patol. oral cir.bucal (Internet) vol.11 no.3 Mai./Jun. 2006
Dental caries in type 1 diabetics: influence of systemic factors of the disease upon the development of dental caries
Caries dental en diabéticos tipo 1: Influencia de factores sistémicos de la enfermedad en la instauración de la caries dental
Lucia Miralles 1, F. Javier Silvestre 2, Antonio Hernández-Mijares 3, Daniel Bautista 1,
Fernando Llambes 4, Diana Grau 1
(1) Associate Professor, University of Valencia (Spain)
(2) Assistant Professor, Department of Stomatology.
University of Valencia Medical and Dental School.
Director of the Stomatology
Unit, Dr Peset University Hospital. Valencia (Spain)
(3) Assistant Professor, Department of Medicine. University
of Valencia Medical and Dental School.
Section Chief, Service of Endocrinology,
Dr. Peset University Hospital. Valencia (Spain).
(4) Doctor of Odontology. Specialist in Periodontics.
Valencia (Spain)
ABSTRACT
Objectives: Diabetes mellitus is one of the most common
chronic diseases in the general population. A study is made of the presence of
caries in type 1 diabetic patients. The specific aim was to determine whether
such patients present a greater incidence of dental caries than non-diabetic
individuals. An evaluation was also made of the relationship of caries to
salivary flow and to factors inherent to the disease such as the degree of
metabolic control, the duration of diabetes, and the existence of chronic
complications.
Study design: The study comprised 90 type 1 diabetics between
18 and 50 years of age, and a group of non-diabetic controls matched for age and
sex. Visual and tactile exploration of the dentition was carried out in all
cases. Oral hygiene was rated based on the OLeary plaque index, and basal (unstimulated)
and stimulated salivary flow were evaluated in both groups. In the diabetic
group, correlations were established with disease control based on the mean
glycosylated hemoglobin (HbA1c) value corresponding to the two years prior to
examination; evolution of the disease in years; and the existence of
complications such as diabetic neuropathy or retinopathy.
Results: Under similar conditions of oral hygiene and
salivary flow, the diabetic group showed a higher incidence of caries than the
control group (p<0.05). Likewise, on specifically analyzing the diabetic group,
metabolic control of the disease, the duration of diabetes, and the existence of
complications of the disease exerted an influence upon the development of dental
caries.
Conclusions: Qualitative salivary studies are advised to
better account for this increased incidence of caries in the diabetic population.
Key words: Dental caries, diabetes mellitus, metabolic control, evolution of diabetes, complications of diabetes.
RESUMEN
Objetivos: La diabetes mellitus es una de las
enfermedades crónicas más prevalentes en población general. Se presenta un
estudio que pretende evaluar la presencia de caries en los pacientes diabéticos
tipo 1. El objetivo específico era comprobar si había o no mayor incidencia de
caries en diabéticos tipo 1 comparándolos con un grupo de individuos no
diabéticos. También, se comprobó la relación con las tasas de flujo salival y
con los factores propios de la enfermedad como el grado de control metabólico,
el tiempo de evolución de la enfermedad y la existencia de complicaciones
crónicas.
Diseño del estudio: Se estudiaron 90 diabéticos tipo 1 de
edades comprendidas entre los 18 y los 50 años de edad y un grupo de pacientes
controles no diabéticos pareados por edad y sexo. Se realizó una exploración
visual y táctil, en todos los dientes de los sujetos explorados. Se tuvo en
cuenta el grado de la higiene oral mediante el índice de placa de OLeary, y se
estudiaron las tasas de saliva basal y estimulada en ambos grupos.
En el grupo diabético se relaciono con el control de la
enfermedad mediante la obtención de la media de la hemoglobina glicosilada (HbA1c)
de los dos años previos a la exploración, así como la evolución de su enfermedad
en años y la existencia de complicaciones como neuropatía diabética o
retinopatía.
Resultados:
Bajo similares condiciones de higiene oral y
de flujo salival, el grupo diabético resultó tener una incidencia de caries
mayor que el grupo control (p<0.05). Asimismo, al estudiar específicamente al
grupo diabético, ni el control metabólico de la enfermedad, ni la evolución de
la enfermedad, ni la existencia de complicaciones de la diabetes tenían
influencia en la instauración de caries dental.
Conclusiones: Se sugiere que habría que estudiar factores
cualitativos salivales que explicasen esta mayor incidencia de caries en los
diabéticos.
Palabras clave: Caries dental, diabetes mellitus, control metabólico, evolución de diabetes, complicaciones de diabetes.
Introduction
The oral health of diabetic patients has been the subject of many studies in recent years. While these patients are acknowledged to have an increased susceptibility to periodontal disease (1,2), the probability of detecting an increased prevalence of dental caries in patients with type 1 diabetes is more controversial (3-5).
On one hand, some authors (3) have reported fewer caries in type 1 diabetic patients relating this observation to the diet prescribed in such patients, with restricted sugar intake. However, other studies have reported an increased presence of caries in diabetic patients attributing this observation to the existence of poorer metabolic control in such individuals (4). In this context, the purported increased risk of dental caries would be related to certain factors such as poor oral hygiene or a lack of blood glucose control. This latter situation could be due to an incorrect diet, or to deficient control of the administered insulin dose with respect to physical exercise of the timing of meals (6-8).
The present study examines whether patients with type 1 diabetes mellitus present a greater incidence of dental caries than non-diabetic individuals, and explores the possible factors underlying such an increased incidence.
Material and methods
The study comprised 90 type 1 diabetics between 18 and 50 years of age, controlled in the Service of Endocrinology (Dr. Peset University Hospital. Valencia, Spain). The exclusion criteria were type 2 diabetes, the presence of other concomitant systemic disorders unrelated to the complications of diabetes, pregnancy, or the use of other medication in addition to insulin. A group of 90 non-diabetic controls matched for age and sex with the diabetic series was also included, comprising persons accompanying patients seen in the Stomatology Unit of the same Hospital. The controls were required to present no systemic diseases, pregnancy or drug use. Informed consent to participation was obtained in all cases.
Following detailed anamnesis, including questioning about disease antecedents, current conditions of the disease and other potentially influencing habits or factors, clinical examination was carried out in the dental chair under conditions of good illumination, in both groups of subjects. Oral lesions were examined using a dental probe, with transparency and percussion testing of all explored teeth. A panoramic X-ray study was also made. The results corresponding to each individual were recorded on an odontogram, with determination of the CAOD index for assessing caried, absent and obturated teeth. Third molars were not considered to the effects of the study. Oral hygiene was rated based on the OLeary plaque index (9). Bacterial plaque was dyed and sampled with a blunt-tipped dental probe, sliding the latter along the gingival sulcus at four points per tooth, and evaluating all surfaces. The presence or absence of plaque was evaluated, regardless of its amount, and the corresponding index was obtained as a percentage on summing the results and dividing by the total number of points explored.
Saliva was in turn collected using the drainage technique (10). The patient first received a series of general instructions. In the hour prior to testing, the patients were required to avoid eating or chewing gum, and were not allowed to smoke. The patients were appointed early in the morning under fasting conditions, and were placed comfortably in the dental chair with the head tilted slightly forward and the lips separated. The patients were instructed to retain the saliva accumulating within the mouth for one minute, after which they were requested to pour the accumulated saliva each minute into a glass funnel connected to a graded test tube, during a total of 5 minutes. Stimulated salivary flow was in turn evaluated after chewing a paraffin tablet for 5 minutes, though pouring the accumulated saliva unto a test tube every minute. In both cases (basal and stimulated saliva output) the tubes containing the saliva were left to stand for a few minutes to clear the foam and thus allow measurement. The amounts obtained were divided by 5 to yield the basal (unstimulated) and stimulated salivary flow rates in milliliters per minute.
In the diabetic group the mean glycosylated hemoglobin value corresponding to the previous year was determined by high performance liquid chromatography (HPLC)(Adams TM Alc HA-8160, Menarini Diagnosticos). Moderate or good disease control in diabetics was considered for HbA1c values of no more than 7.5%, while in normal (control) subjects the value was taken to be 6% or lower. On the other hand, the evolution of the disease (in years) was recorded, along with any antecedents of microvascular complications directly associated with the disease such as diabetic retinopathy, neuropathy or nephropathy.
Statistical analysis
Quantitative variables were expressed as the mean and standard deviation (SD), while qualitative variables were reported as frequencies. Correlations between variables were examined using the Spearman correlation test for both metric and nonparametric variables. The qualitative variables were analyzed using the chi-square test, with application of the Student t-test for quantitative variables, after confirming normal distribution with the Shapiro-Wilkes test. The Student t-test for independent samples was used, accepting statistical significance for p<0.05. The Mann-Whitney U-test was in turn applied to samples without a normal distribution.
Results
The mean CAO index was significantly higher in the group of diabetic patients (7.41±4.17) than in the controls (5.63±4.04), with a greater caries index among the former (Student t-test for independent samples) (Table 1).
The OLeary plaque index was similar in both groups: 63.34% among the diabetics versus 63.17% in the control group.
Basal salivary flow showed no significant intergroup differences, with a mean value of 0.29±0.28 ml/min. versus 0.33±0.21 ml/min. in the control group. Similar considerations applied to stimulated salivary flow: 0.99±0.54 ml/min. among the diabetics versus 0.95±0.53 ml/min. in the control group.
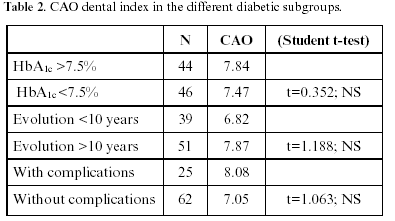
On examining the possible variables influencing the appearance of caries in patients with type 1 diabetes mellitus (Table 2), we first considered the effects of patient metabolic control. Of the 90 diabetic patients studied, 44 were found to be decompensated in terms of glycosylated hemoglobin, with HbA1c >7.5%, while in the remaining 46 diabetics the value was ≤7.5%. On considering the mean CAO index for the patients with HbA1c >7.5% (i.e., decompensated diabetics), the value was found to be 7.84±4.48, versus 7.47±4.29 among the patients with good to moderate control. This difference was not statistically significant thus suggesting that disease control on the basis of HbA1c is not related to the appearance of dental caries.
Likewise, we studied the possible influence of the evolution of the disease upon the appearance of caries. In this sense, the recorded mean CAO index for the 39 patients with a duration of diabetes of less than 10 years was 6.82±3.9, versus 7.87±4.19 among those who had suffered the disease for more than 10 years. This difference likewise failed to reach statistical significance, i.e., no relationship was observed between the incidence of caries and the duration of diabetes in this study group.
Lastly, on evaluating whether the existence of complications inherent to diabetes such as retinopathy, nephropathy or neuropathy exert an influence upon the high caries index observed among the diabetic population, we found that the 25 patients with complications exhibited a CAO index of 8.08±3.92. In comparison, the 62 diabetics without complications of the disease presented a caries index of 7.05±4.16. While this figure was lower than in the subgroup of patients with complications, no significant relationship was recorded between the presence or absence of complications inherent to diabetes and the corresponding patient caries index.
Discussion
The clinical results of the present study indicate an increased vulnerability to dental caries in patients with type 1 diabetes compared with non-diabetic individuals. Dental caries is an infectious disorder involving multiple factors that coincide at a given point and at a given time. The basic factors are the presence of the causal microorganism, the host (tooth), substrate (diet) and immune capacity of the patient. The microorganisms associated with dental caries were studied by Twetman et al. (11) in diabetic patients. These authors reported a high proportion of Streptococcus mutans in the aerobic flora of the oral cavity. Other authors (12,13) have reported a decrease in the presence of salivary lactobacilli, due to the diet prescribed in such patients. In contrast, other authors such as Iughetti et al. (3) have observed similar counts of Streptococcus mutans and lactobacilli in diabetic and non-diabetic patients. Increased bacterial plaque aggregation has also been related to the saliva of these patients (14).
Moore et al. (15) reported an increased prevalence of caries in type 1 diabetics, located particularly in the root or dental neck regions. The authors related this observation to the age of the patients, the presence of increased gingival recession, and the presence of concomitant kidney disease. Caries of this kind has also been related to a specific diet based on carbohydrates, with an incorrect provision of calcium and phosphorus (16). However, this is in contrast to the fact that patients with good diabetes control have reduced sugar intakes (17).
Other authors have related the increased prevalence of caries to the degree of metabolic control among the patients, and the existence of complications inherent to diabetes (18). In the present study we were unable to link the number of caries to the patients with poorer metabolic control or disease complications.
Diabetic patients are known to tend to develop caries in concrete locations. In this sense, saliva has been identified as a factor related to the appearance of dental neck caries (19-22). However, xerostomia in a diabetic patient may actually refer to dry mouth sensation secondary to peripheral neuropathy, rather than to genuinely diminished saliva output (23). In the present study we likewise found no differences in basal and stimulated salivary flow between the diabetic patients and healthy controls. This coincides with the results of an earlier study involving a sample of young type 1 diabetics (24). Likewise, we observed no differences between the two groups in terms of bacterial plaque. As suggested by Belazi et al. (25), qualitative factors should be explored, such as increased concentrations of glucose in the saliva of diabetic patients, or immune factors contained in saliva.
In coincidence with Arrieta-Blanco et al. (26), we conclude that type 1 diabetics have an increased incidence of caries, though this phenomenon could not be related to metabolic control, the duration of the disease, or the appearance of late complications of diabetes. Moreover, and unlike these authors, we were also unable to link caries to oral hygiene.
![]() Correspondence:
Correspondence:
Prof. F. J. Silvestre
Clínica Odontológica
Gascó Oliag, 1
46021 Valencia
E-mail: francisco.silvestre@uv.es
Received: 20-11-2005
Accepted: 25-03-2006
References
1. Seppala B, Seppala M, Ainamo J. A longitudinal study on insulin-dependent diabetes mellitus and periodontal disease. J Clin Periodontol 1993;20:161-5. [ Links ]
2. Mattson JS, Cerulis DR. Diabetes mellitus: A review of the literature and dental implications. Compendium 2001;22:757-72. [ Links ]
3. Iughetti L, Marino R, Bertolani MF, Bernasconi S. Oral health in children and adolescents with IDDM. A review. J Pediatr Endocrinol Metab 1999;12:603-10. [ Links ]
4. Canepari P, Zerman N, Cavalleri G. Lack of correlations between salivary Streptococcus mutans and lactobacilli counts and caries in IDDM children. Minerva Stomatol 1999;43:501-5. [ Links ]
5. Twetman S, Johansson I, Birkhed D, Nederfors T. Caries incidence in young type 1 diabetes mellitus patients in relation to metabolic control and caries-associated risk factors. Caries Res 2002;36:31-5. [ Links ]
6. Syrjälä A-MH, Ylöstalo P, Niskanen MC, Knuuttila MLE. Relation of different measures of psychological characteristics to oral health habits, diabetes adherence and related clinical variables among diabetics. Eur J Oral Sci 2004;112:109-14. [ Links ]
7. Siudikiene J, Maciulskiene V, Dobrovolskiene R, Nedzelskiene I. Oral hygiene in children with type I diabetes mellitus. Stomatologija 2005;7: 24-7. [ Links ]
8. Siudikiene J, Maciulskiene V, Nedzelskiene I. Dietary and oral hygiene habits in children with type I diabetes mellitus related to dental caries. Stomatologija 2005;7:58-62. [ Links ]
9. OLeary TJ, Drake RB, Naylor JE. The plaque control record. J Periodontol 1972;43:38. [ Links ]
10. López-Jornet MP. Principales técnicas de recogida y registro del fluido salival en el hombre. Ventajas e inconvenientes. Murcia: Universidad de Murcia; 1993. p. 11-3. [ Links ]
11. Twetman S, Aronsson S, Bjorkman S. Mutans streptococci and lactobacilli in saliva from children with insulin dependent diabetes mellitus. Oral Microbiol Inmunol 1989;4:165-8. [ Links ]
12. Swanljung O, Meurman JH, Torkko H, Sandholm L, Kaprio E, Maenpaa J. Caries and saliva in 12-18 years old diabetics and controls. Scand J Dent Res 1992;100:310-3. [ Links ]
13. Collin HL, Uusitupa M, Niskanen L, Koivisto AM, Markanen H, Meurman JH. Caries in patients with non insulin dependent diabetes mellitus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1998;85:680-5. [ Links ]
14. Anderson LC, Yang SC, Xie H, Lamont RJ. The effects of streptozotocin diabetes on salivary-mediated bacterial agregation and adherence. Arch Oral Biol 1994;39:261-9. [ Links ]
15. Moore PA, Weyant RJ, Etzel KR, Guggenheimer J, Mongelluzzo MB, Myers DE et al. Type 1 diabetes mellitus and oral health: assesment of coronal and root caries. Community Dent Oral Epidemiol 2001;29:183-94. [ Links ]
16. Ciglar L, SKaljac G, Sutalo J, Keros J, Jankovic B, Knezevic A. Influence of diet on dental caries in diabetics. Coll Antropol 2002;26:311-7. [ Links ]
17. Tavares M, Depaola P, Soparkar P, Joshipura K. The prevalence of root caries in a diabetic population. J Dent Res 1991;70:979-83. [ Links ]
18. Bullón P, Machuca G. Tratamiento odontológico en pacientes especiales. 2ª ed. Madrid: Normon; 2004. p. 511-31. [ Links ]
19. Twetman S, Nederfors T. Two year longitudinal observations of salivary status and dental caries in children with insulin dependent diabetes mellitus. Pediatric Dentistry 1992;14:184-9. [ Links ]
20. Lin B, Taylor GW, Allen DJ, Ship JA. Dental caries in older adults with diabetes mellitus. Spec Care Dentist 1999;19:8-14. [ Links ]
21. Karjalainen KM, Knuuttila MLE, Käär ML. Relationship between caries and level of metabolic balance in children ad adolescents with insulin dependent diabetes mellitus. Caries Res 1997;31:13-8. [ Links ]
22. Moore PA, Weyant RJ, Mongelluzzo MB, Myers DE, Rossie K, Guggenheimer J et al. Type 1 diabetes mellitus and oral health: assesment of tooth loss and edentulism. J Public Oral Health 1998;58:135-42. [ Links ]
23. López-Jornet P, Saura-Inglés A, Martínez-Mondéjar B, Bermejo-Fenoll A. Valoración de la tasa de flujo salival en pacientes con diabetes mellitus tipo 1 y tipo 2. Archivos de Odontoestomatol 1996;12:690-7. [ Links ]
24. Miralles L, Silvestre FJ, Grau DM, Hernández A. Estudio clínico sobre la patología bucodentaria en el paciente diabético tipo 1. Medicina Oral 2002;7:298-302. [ Links ]
25. Belazi MA, Galli-Tsinopoulou A, Drakoulakos D, Fleva A, Papanayiotou PH. Salivary alterations in insulin- dependent diabetes mellitus. Int J Paediatr Dent 1998;8:29-33. [ Links ]
26. Arrieta-Blanco JJ, Bartolomé-Villar B, Jiménez-Martinez E, Saavedra-Vallejo P, Arrieta-Blanco FJ. Problemas bucodentales en pacientes con diabetes mellitus (I): Índice de placa y caries dental. Med Oral 2003; 8:97-109. [ Links ]











 texto em
texto em