Meu SciELO
Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Medicina Oral, Patología Oral y Cirugía Bucal (Internet)
versão On-line ISSN 1698-6946
Med. oral patol. oral cir.bucal (Internet) vol.11 no.5 Ago./Set. 2006
ORAL MEDICINE AND PATHOLOGY
Bisphosphonates and Oral Pathology I. General and preventive aspects
Bisfosfonatos y Patología Oral I. Aspectos generales y preventivos
Nerea Ponte Fernández1, Ruth Estefania Fresco1, José Manuel Aguirre Urizar1
(1) Medicina Bucal. Departamento de Estomatología. Unidad de Patología Oral y
Maxilofacial.
Servicio Clínica Odontológica. Universidad del País Vasco EHU
ABSTRACT
Bisphosphonates constitute a group of drugs capable of modulating bone turnover, and reduce its remodelling when an excessive resorption occurs. This is why they are indicated in a large group of bone diseases like postmenopausal osteoporosis or osteolisis associated with breast cancer or multiple myeloma. Over the last years and due to their extensive use, many cases of complications associated with their use have been published. Among the most important possible adeverse effects are the oral ones, with the appearance of ulcerations and, especially, osteonecrosis of the jaws associated with this therapy. In this paper, we have analyzed the general characteristics of these drugs and their mechanisms of action as well as the described adverse effects, especially oral and maxillofacial has been made special reference regarding the prevention of osteonecrosis of the jaws, hightened by cases described in medical and odontological literature. The preventive protocol backs up the fundamental role of the odontologist in the effective prevention of this process before, during and after the treatment.
Key words: Bisphosphonates, adverse effects, osteonecrosis, jaws, prevention.
RESUMEN
Los bisfosfonatos constituyen un grupo de fármacos capaces de modular el recambio óseo y disminuir su remodelado cuando existe una reabsorción excesiva. Por ello están indicados en numerosas patologías óseas como la osteoporosis postmenopáusica o la osteolisis asociada al cáncer de mama o al mieloma múltiple. En los últimos años y a raíz de su utilización masiva se han ido publicando numerosos casos de complicaciones asociadas a su uso. Entre los posibles efectos adversos más importantes se encuentran los orales, con la aparición de ulceraciones y sobre todo los casos de osteonecrosis de los maxilares asociados a esta terapéutica. En esta revisión analizamos las características generales de estos medicamentos y su mecanismo de actuación, así como los efectos adversos descritos, especialmente los orales y maxilofaciales, haciendo una referencia especial sobre la prevención de la osteonecrosis de los maxilares, a la luz de los casos descritos en la literatura médica y odontológica. El protocolo preventivo refuerza el papel fundamental del odontólogo en la prevención efectiva de este proceso antes, durante y después del tratamiento.
Palabras clave: Bisfosofonatos, efectos adversos, osteonecrosis, maxilar, prevención.
General aspects
Bisphosphonates (BPP) are non metabolized analogues of pyrophosphate, capable of binding to the bone and inhibit the osteoclast function reducing bone turnover and the active remodelation in places where excessive bone reabsorption occurs (1). They have been used in toothpaste as antiplaque and in diagnostic tests as a conveyor of Tc 99(2).
The first BPP introduced was etidronate with low power and with a tendency to produce osteomalacia (3). Searching for more powerful compounds and without adverse effects, the second (alendronate, pamidronate, ibandronate, tiludronate) and the third generation BPP risedronate, zoledronic acid, minodronate) have been developed.
Few changes in the structure of BPP modify their physicochemical, biological, therapeutic and toxicological properties (4).
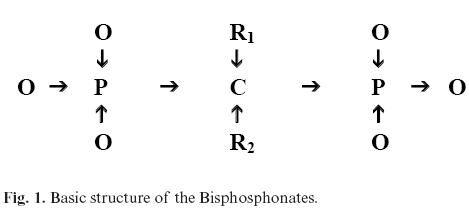
The structure of BPP (Figure 1), shows two phosphate groups binding to a carbon atom (P-C-P), that to a certain extent is responsible for their low bioavailability. Like pyrophosphates, BPP bind strongly to hidroxiapatite, explaining their pharmacological actions in the bone (5). R1 radicals influence in their affinity, therefore, OH groups increase the fixation, while Cl groups decrease it. This would explain why BPP with OH groups (alendronate, pamidronate ) bind more than clodronate (6).
BPP are released when there is bone reabsorption that would explain their long half-life (7). This release is multiphasic, and in the case of alendronate is 10.5 years. This fact would explain their persistent long term effect in the bone (8).
Mechanism of action
BPP inhibit bone reabsorption by reducing osteoclast reabsorptive activity (9), promoting their apoptosis (10), avoiding their formation from hematopoyetic precursors (11) or affecting osteoblasts (12).
Once inside the osteoclast, R2 chain determines the power and efficacy of the drug. In the case of clodronate (Cl in R2) and etidronate (CH3 in R2), they act through the metabolization to toxic analogues of ATP that induce osteoclastic apoptosis (13).
Different studies (14,15), have proved the role of FPP-synthase like molecular target of nitrogen-containing BPP. These drugs induce osteoclast apoptosis by the inhibition of cholesterol biosynthesis and the mevalonate pathway, due to the inhibition of the synthesis of farnesilpirophosphate (16-19). These disturbances affect the organization of cytoskeleton, vesicle traffic and the formation of the osteoclast brush border. BPP also have some effect on the osteoblasts, which is still not totally clear, reducing apoptosis and stimulating the secretion of inhibitors in the recruitment of osteoclasts (20).
It has also been said to have an antiangiogenic effect on BPP by the inhibition of endothelial cells, reducing their proliferation and inducing apoptosis (21).
Therapeutic indications
The indications of BPP have undergone a large evolution since they were introduced in the treatment of some bone and calcium metabolic disorders (22, 23). Nowadays, the main indications of BPP are:
1. In postmenopausal and corticosteroid induced osteoporosis. In these cases the most used BPP is alendronate, which precludes the appearance of pathological fractures (24).
2. In Paget's disease, it is used to improve bone morphology and reduce pain (25).
3. In hypercalcaemia of malignancy, its role is in trying to check hypercalcaemia, reducing pain and preventing the development of osteolitic lesions and fractures (26).
4. In patients with breast and prostate cancer with bone metastasis, it is used to relieve pain, reduce fractures and hypercalcaemia (27, 28).
5. In multiple myeloma, it is indicated to reduce associated bone pathologies such as vertebral collapse, fractures and pain (29, 30).
Oral BPP are potent inhibitors of osteoclasts, but less efficient in the treatment of bone processes associated with malignant diseases. Therefore, they were principally indicated in the treatment of osteoporosis. On the other hand, systemic BPP are indicated in patients with metastatic breast cancer, multiple myeloma, hypercalcaemia of malignancy, Pagets disease and bone metastasis of solid tumours (prostate, lung )
General adverse effects
BPP are well tolerated in general if they are correctly administrated. Nevertheless, various adverse effects and complications associated with their use have been described.
In the case of oral BPP, the digestive effects are the most frequently described: gastric ulcerations, oesophagitis and oesophago stenosis.
Isolated cases of uveitis in patients treated with alendronate (31, 32), pamidronate (33, 34) and recently with zoledronic acid (35) have been described.
The continuous administration of etidronate can produce a picture of osteomalacia that regresses after discontinuation of therapy (36, 37). Nevertheless, the most modern BPP do not present this adverse effect.
Some experimental models have contemplated the possibility that the inhibition of bone remodelation may lead to a reduction of bone resistance although there is no clinical evidence, at least concerning the administration of oral BPP. In fact, even with the most powerful BPP, the remodeling process is completely annulled (38).
The general adverse effects of intravenous BPP are similar to the oral ones, and some cases of flebitis (18%) have been described. There are also accounts of transitory febricula, shivers (10-41%) and a pseudogrippal syndrome (20%) in the first two days (39).
There are references of hypocalcaemia, usually asymptomathic, after high doses of BPP (40, 41). Nevertheless, most of the patients are unaffected thanks to the compensatory increase of parathyroid hormone (42).
One has to be careful when high doses of intravenous BPP are administrated because its rapid administration can cause renal failure, due to the formation of a solid phase in the blood, which is retained in the kidney.
Oral adverse effects
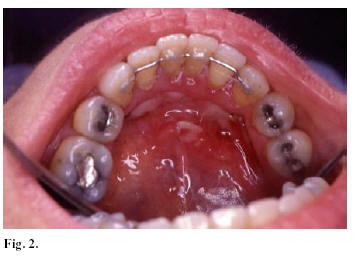
There are some cases of chronic ulcerations in the oral mucosa associated with the administration of aldosterone in osteoporotic patients (44). These cases belonged to postmenopausal osteoporotic women in treatment with alendronate. They presented oral ulcerations in the mucosa secondary to an inappropriate administration, which disappeared with topic corticoids. There are other cases of oral ulcerations generally associated with an inappropriate administration (45, 46). Figure 2 presents a recent case of a 55 year woman with ulcerations in the floor of the mouth associated with the alendronate administration.
In the past 4 years there have been publications describing numerous cases of a special form of osteonecrosis of the jaws (ONJ) in patients affected with myeloma or breast cancer and in treatment with high effectiveness BPP, especially pamidronate and zoledronic acid and more rarely with alendronate (47-50).
Two theories have been proposed to explain the appearance of ONJ. One related to the action of the BPP in bone turnover, and the other to their antiangiogenic action (51). The main theory suggests that ONJ is caused by the cessation of bone remodelling and the osteoclast-inhibiting effect. BPP is highly concentrated in the jaws because there is a greater blood supply than in other bones and a faster bone turnover, related to their high activity and the presence of the teeth. This circumstance, together with the frequent appearance of dental pathologies, odontological treatment and the thin mucosa over the maxilar bone; explains why osteonecrosis is especially patent in these bones.
The bone turnover maintenance is crucial to maintain bone viability. If osteoclast function decreases severely, osteocytes are not replaced and bone capillary network is not maintained, permitting the appearance of avascular bone necrosis (52). The breakdown of the oral mucosa, caused by a traumatic ulceration or surgery, causes local bone necrosis which progresses when healing fails. The risk of ONJ increases with dental manipulation and poor hygiene, because bone is exposed to the oral microbiota, later causing infection with pain, tumefaction, suppuration and progressive bone necrosis, which is difficult to control (53).
The other etiopatogenic theory is based on experimental evidence showing that most potent BPP also inhibit capillary neoangiogenesis, decreasing capillary formation and inhibiting endothelial growth factors (21), leading to avascular necrosis. However, this is not the only cause as it is not associated with the administration of more potent antiangiogenic drugs (54, 55).
There are accounts of ONJ cases associated with BPP in patients who have been on medication for years, and even for a few weeks (53). However, a study of multiple myeloma patients showed that ONJ risk is time-dependent and is significant only after 12 or 36 months after (56).
Prevention of BPP associated osteonecrosis of the jaws
As in any prophylactic approach, the preventative measures of osteonecrosis of the jaws is introduced before initiating BPP therapy.
a.- Measures before initiating BPP therapy
As soon as the oncologist or other specialists consider the administration of BPP necessary, the patient should be referred to a dentist for an urgent oral examination (57), consisting of a complete clinical and radiographic exploration.
Oral treatment is aimed at eliminating infections and the need for invasive dental procedures in the near future; so preventive therapy should be aggressive and should include: tooth removal, periodontal surgery, root canal treatment, tooth decay control, dental restorations and prothesis if needed. These patients are not candidates for dental implants because of the risk elements involved (51). A month before initiating BPP therapy, removal of large mandibular tori or palatal tori with thin overlying mucosa, is recommended to avoid the risk of ulcerations.
Patients with no cardiac disease do not require prophylactic antibiotic coverage for non invasive procedures but there is a need for an invasive procedure; in this case, the chosen drug is penicillin and its derivatives. For individuals with penicillin allergy, a combination therapy using quinolones and metronidazole have proven efficient. Clindamycin alone is not recommended because it is ineffective against actinomyces, Eikenella corrodens, and similar species that frequently colonize the exposed bone in the oral cavity (51).
If the patient requires only non invasive dental care BPP therapy is not delayed. But if the patient requires invasive procedures, BPP therapy should be deferred for a month to allow bone recovery and good healing. A surveillance schedule every 4 months with plaque control and strict hygienic measures is recommended.
b.- Measures during BPP therapy
The dentist should carefully examine the oral cavity in the search for the presence of bone expositions in the most commonly affected areas, such as the posterior and lingual area of the mandible. A complete radiographic examination should be carried out to look for signs of osteolysis, osteosclerosis, widened periodontal spaces and furcation affection.
A careful dental cleaning and preventive use of fluor and clorhexidine should be considered as well as strict hygienic measures (51).
All the needed procedures to conserve the teeth and avoid the appearance of infections should be done as soon as possible. If the tooth is not restorable, root canal treatment and amputation of the crown are preferable to extraction. Root canal treatment should be carried out with minimum trauma to the marginal and apical periodontal tissues (58).
In relation to periodontal disease, teeth will only be removed when they have 3 or more mobility grade or an associated periodontal abscess. In these situations, adequate antibiotic treatment is imperative, as previously indicated (51).
Surgery procedures are contraindicated. Nevertheless, in patients that need surgery, BPP withdrawal is recommended in the case of a bad recovery. However, it is not certain that BPP withdrawal will prevent the appearance of ONJ, due to their half-life (59).
When oral surgery procedure is needed in a patient with BPP therapy, some special precautions should be taken as for instance, absolutely aseptic procedures, atraumatic surgery and achieving a first intention closing if possible (60).
If prosthesis is needed, it should be preferably fixed and well carried out, to avoid if possible the appearance of secondary traumatic ulcerations.
In patients in treatment with BPP it is recommended to engage in a continuous surveillance schedule every 3 or 4 months with plaque control and strict hygienic measures.
Acknowledgements: We want to thank to Mr. David Hallett for revising the English language in this paper.
![]() Address for correspondence:
Address for correspondence:
Prof. José Manuel Aguirre Urízar
Medicina Bucal. Departamento de Estomatología.
Universidad del País Vasco EHU
Barrio Sarriena s/n
Leioa 48940. Vizcaya
E-mail: otpagurj@lg.ehu.es
Received: 29-03-2006
Accepted: 26-06-2006
References
1. Rodan GA, Reszka AA. Bisphosphonate mechanism of action. Curr Mol Med 2002;2:571-7. [ Links ]
2. Mellibovsky L. Osteoporosis, bisfosfonatos y calcitonina. JANO 2001; 61:47-54. [ Links ]
3. Fleish H. Bisphosphonates: Mechanisms of Action. Endocr Rev 1998; 19:80-100. [ Links ]
4. Fleish H. The role of bisphosphonates in breast cancer: Development of bisphosphonates. Breast Cancer Res 2002; 4: 30-4. [ Links ]
5. Jung, A, Bisaz S, Fleisch H. The binding of pyrophosphate and two diphosphonates on hydroxyapatite crystals. Calcif Tissue Res 1973;11: 269-80. [ Links ]
6. Shinoda H, Adamek G, Felix R, Fleisch H, Schenk R, Hagan P. Structure-activity relationships of various bisphosphonates. Calcif Tissue Int 1983;35:87–99. [ Links ]
7. Kasting GB, Francis MD. Retention of etidronate in human, dog, and rat. J Bone Miner Res 1992; 7: 513-22. [ Links ]
8. Khan SA, Kanis JA, Vasikaran S, Kline WF, Matuszewski BK, McCloskey EV et al. Elimination and biochemical responses to intravenous alendronate in postmenopausal osteoporosis. J Bone Miner Res 1997; 12:1700-7. [ Links ]
9. Flanagan AM, Chambers TJ. Inhibition of bone resorption by bisphosphonates: Interactions between bisphosphonates, osteoclasts, and bone. Calcif Tissue Int 1991;49:407-15. [ Links ]
10. Hughes DE, Wright KR, Uy HL, Sasaki A, Yoneda T, Toodman GD et al. Bisphosphonates promote apoptosis in murine osteoclasts in vitro and in vivo. J Bone Miner Res 1995;10:1478-87. [ Links ]
11. Boonekamp PM, van der Wee-Pals LJ, van Wijk-van Lennep MM, Thesing CW, Bijvoet OLM. Two modes of action of bisphosphonates on osteoclastic resorption of mineralized matrix. Bone Miner 1986;1:27-39. [ Links ]
12. Sahni M, Guenther HL, Fleisch H, Collin P, Martin TJ. Bisphosphonates act on rat bone resorption through the mediation of osteoblasts. J Clin Invest 1993;91:2004-11. [ Links ]
13. Frith JC, Monkkonen J, Auriola S, Monkkonen H, Rogers MJ. The molecular mechanism of action of the antiresorptive and antiinflammatory drug clodronate: evidence for the formation in vivo of a metabolite that inhibits bone resorption and causes osteoclast and macrophage apoptosis. Arthritis Rheum 2001;44:2201-10. [ Links ]
14. Ciosek CP, Magnin DR, Harrity TW, Logan JV, Dickson JK, Gordon EM et al. Lipophilic 1,1-bisphosphonates are potent squalene synthase inhibitors and orally active cholesterol lowering agents in vivo. J Biol Chem 1993;268:24832-7. [ Links ]
15. Fisher JE, Rodan GA, Reszka AA. In vivo effects of bisphosphonates on the osteoclast mevalonate pathway. Endocrinology 2000;141:4793-6. [ Links ]
16. Luckman SP, Coxon FP, Ebetino FH, Russell RGG, Rogers MG. Heterocycle-containing bisphosphonates cause apoptosis and inhibit bone resorption by preventing protein prenylation: evidence from structure-activity relationships in J774 macrophages. J Bone Miner Res 1998; 13:1668-78. [ Links ]
17. Luckman SP, Hughes DE, Coxon FP, Russell RGG, Rogers MG. Nitrogen-containing bisphosphonates inhibit the mevalonate pathway and prevent post-translational prenylation of gtp-binding proteins, including ras. J Bone Miner Res 1998;13:581-9. [ Links ]
18. van Beek E, Pieterman E, Cohen L, Löwik C, Papapoulos S. Farnesyl pyrophosphate synthase is the molecular target of nitrogen-containing bisphosphonates. Biochem Biophys Res Commun 1999;264:108-11. [ Links ]
19. Bergstrom JD, Bostedor RG, Masarachia PJ, Rewszka AA, Rodan GA. Alendronate is a specific, nanomolar inhibitor of farnesyl diphosphate synthase. Arch Biochem Biophys 2000;373:231-41. [ Links ]
20. Fleish HA. Bisphosphonates: preclinical aspects and use in osteoporosis. Ann Med 1997;29:55-62. [ Links ]
21. Fournier P, Boissier S, Filleur S, Guglielmi J, Cabon F, Colombel M et al. Bisphosphonates inhibit angiogenesis in vitro and testosterona-stimulated vascular regrowth in the ventral prostate in castrated rats. Cancer Res 2002;62:6538-44. [ Links ]
22. Green JR, Rogers MJ. Pharmacologic profile of zoledronic acid; a highly potent inhibitor of bone resorption. Drug Rev Res 2002; 55: 210-44. [ Links ]
23. Lenz JH, Steiner-Krammer B, Schmidt W, Fietkau R, Mueller PC, Gundlach KK. Does avascular necrosis of the jaws in cancer patients only occur following treatment with bisphosphonates?. J Cranio-Maxillofac Surg 2005;33:395-403. [ Links ]
24. Bagger YZ, Tanko LB, Alexandersen P, Ravn P, Christiansen C. Alendronate has a residual effect on bone mass in postmenopausal danish women up to 7 years after treatment withdrawal. Bone 2003;33:301-7. [ Links ]
25. Reid IR, Miller P, Lyles K, Fraser W, Brown JP, Saidi Y et al. Comparison of a single infusion of zoledronic acid with risedronate for Pagets disease. N Engl J Med 2005;353:898-908. [ Links ]
26. Berenson JR, Lichtenstein A, Porter L, Dimopoulos MA, Bordoni R, George S et al. Efficacy of pamidronate in reducing skeletal events in patients with advanced multiple myeloma. N Engl J Med 1996;334: 488–93. [ Links ]
27. Michigami T, Hiraga T, Williams PJ, Niewolna M, Nishimura R, Mundy GR et al. The effect of the bisphosphonate ibandronate on breast cancer metastasis to visceral organs. Breast Cancer Res Treat 2002;75:249-58. [ Links ]
28. Bauss F, Body JJ. Ibandronate in metastatic bone disease: a review of preclinical data. Anticancer Drugs 2005;16:107-8. [ Links ]
29. Berenson JR, Rosen LS, Howell A, Porter L, Coleman RE, Morley W et al. Zoledronic acid reduces skeletal-related events in patients with osteolytic metastases. Cancer 2001;91:1191-200. [ Links ]
30. Roodman GD. Mechanisms of bone metastasis. N Engl J Med 2004; 350: 1655-64. [ Links ]
31. Mbekeani JN, Slamovits TL, Schwartz BH, Sauer HL. Ocular inflammation associated with alendronate therapy. Arch Ophthalmol 1999; 117:837–8. [ Links ]
32. Malik AR, Campbell SH, Toma NM. Bilateral acute anterior uveitis after alendronate. Br J Ophthalmol 2002;86:1443. [ Links ]
33. Ghose K, Waterworth R, Trolove P, Highton J. Uveitis associated with pamidronate. Aust N Z J Med 1994;24:320. [ Links ]
34. Fraunfelder FW, Fraunfelder FT, Jensvold B. Scleritis and other ocular side effects associated with pamidronate disodium. Am J Ophthalmol 2003;135:219–22. [ Links ]
35. Durnian JM, Olujohungbe A, Kyle G. Bilateral acute uveitis and conjunctivitis after zoledronic acid therapy. Eye 2005;19:221–2. [ Links ]
36. Jowsey J, Riggs BL, Kelly PJ, Hoffman DL, Bordier P. The treatment of osteoporosis with disodium ethane-1-hydroxy-1,1-diphosphonate. J Lab Clin Med 1971;78:574–84. [ Links ]
37. Boyce BF, Smith L, Fogelman I, Johnston E, Ralston S, Boyle IT. Focal osteomalacia due to low-dose diphosphonate therapy in Pagets disease. Lancet 1984;1:821–4. [ Links ]
38. Riancho JA. Bifosfonatos orales en el tratamiento de la osteoporosis. En: Riancho JA, González J. Manual Práctico de osteoporosis y enfermedades del metabolismo mineral. Madrid: Jarpyo Editores; 2004 . p. 184-5. [ Links ]
39. Hernández JL. Bifosfonatos intravenosos y osteoporosis. En: Riancho JA, González J. Manual Práctico de osteoporosis y enfermedades del metabolismo mineral. Madrid: Jarpyo Editores; 2004. p. 190. [ Links ]
40. McIntyre E, Bruera E. Symptomatic hypocalcaemia after intravenous pamidronate. J Palliat Care 1996;12:46-7. [ Links ]
41. Mishra A, Wong L, Jonklaas J. Prolonged symptomatic hypocalcaemia with pamidronate administration and subclinical hypoparathyroidism. Endocrine 2001;14:159-64. [ Links ]
42. Fraser WD, Logue FC, Gallacher SJ, OReilly DS, Beastall GH, Ralston SH et al. Direct and indirect assessment of the parathyroid hormone response to pamidronate therapy in Pagets disease of bone and hypercalcemia of malignancy. Bone Miner 1991;12:113-21. [ Links ]
43. Bounameaux HM, Schifferli J, Montani JP, Jung A, Chatelanat F. Renal failure associated with intravenous diphosphonates. Lancet 1983;1:471. [ Links ]
44. González-Moles MA, Bagán-Sebastián JV. Alendronate-related oral mucosa ulcerations. J Oral Pathol Med 2000;29:514-8. [ Links ]
45. Mundy GR, Guise TA. Hypercalcemia of malignancy. Am J Med 1997;103:134-45. [ Links ]
46. Schmutz JL, Barbaud A, Trechot P. Alendronate and mouth ulcers: careful interrogation of the patient to start with. Ann Dermatol Venereol 2005;132:930. [ Links ]
47. Marx RE. Pamidronate (Aredia) and zolendronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. J Oral Maxillofac Surg 2003;61:1115-7. [ Links ]
48. Jiménez Y, Bagán JV. Bisphosphonates, as a new cause of drug-induced jaw osteonecrosis: an update. Med Oral Patol Oral Cir Bucal 2005; 10:88-91. [ Links ]
49. Bagán JV, Murillo J, Jiménez Y, Poveda R, Milian MA, Sanchos JM et al. Avascular jaw osteonecrosis in association with cancer chemoterapy: series of 10 cases. J Oral Pathol Med 2005;34:120-3. [ Links ]
50. Pastor-Zuazaga D, Garatea-Crelgo J, Martino-Gorbea R, Etayo-Pérez A, Sebatián-Lopez C. Osteonecrosis maxilar y bisfosfonatos. Presentación de tres nuevos casos. Med Oral Patol Oral Cir Bucal 2006;11:57-60 [ Links ]
51. Marx RE, Sawatari Y, Fortin M, Broumand V. Bisphosphonate-induced exposed bone (osteonecrosis/osteopetrosis) of the jaws: Risk factors, recognition, prevention, and treatment. J Oral Maxillofac Surg 2005;63:1567-75. [ Links ]
52. Carter G, Goss AN, Doecke C. Bisphosphonates and avascular necrosis of the jaw: a possible association. Med J Aust 2005;182:413-5. [ Links ]
53. Migliorati CA. Bisphosphanates and oral cavity avascular bone necrosis. J Clin Oncol 2003;21:4253-4. [ Links ]
54. Migliorati CA. Bisphosphonate-associated oral osteonecrosis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2005;99:135. [ Links ]
55. Stinchcombe TE, Mitchell BS, Depcets-Smith N, Orlowski RZ, Shea TC, Baldwin AS et al. PS-341(Bortazomib) is active in multiple myeloma. Preliminary report of a phase I trial of the proteasome inhibitor PS-341 in patients with hematologic malignancies. Blood 2000;96:516. [ Links ]
56. Brian GM, Durie MD. Zometa and osteonecrosis of the jaws a new update. Jul 2004. htpp://myeloma.org/main.jsp.aredia/ [ Links ]
57. Greenberg MS. Intravenous bisphosphonates and osteonecrosis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2004;98:259-60. [ Links ]
58. Katz H. Endodontic implications of bisphosphonate-associated osteonecrosis of the jaws: a report of three cases. J Endod 2005;31:831-4. [ Links ]
59. Berenson JR. Recommendations for zoledronic acid treatment of patients with bone metastases. Oncologist 2005;10:52-62. [ Links ]
60. Robinson NA, Yeo JF. Bisphosphonates a word of caution. Ann Acad Med Singapore 2004;33:48-9. [ Links ]











 texto em
texto em