Meu SciELO
Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Pharmacy Practice (Granada)
versão On-line ISSN 1886-3655versão impressa ISSN 1885-642X
Pharmacy Pract (Granada) vol.4 no.3 Redondela Jul./Set. 2006
|
Original research |
Evaluation of anti-tuberculosis induced adverse reactions in hospitalized patients
Evaluación de las reacciones adversas de los antituberculosos en pacientes hospitalizados
Kheirollah GHOLAMI, Elahe KAMALI, Mahboubeh HAJIABDOLBAGHI, Gloria SHALVIRI.
|
ABSTRACT Background: Tuberculosis has been one of the common diseases of human
communities. Besides of disease-related complications, there are serious
adverse reactions due to Anti-tuberculosis (Anti-TB) drug therapy. Key words: Tuberculosis. Inpatients. Adverse Drug Reaction Reporting Systems. Drug Toxicity. Iran. |
RESUMEN Antecedentes: La tuberculosis ha sido una de las enfermedades comunes
de la humanidad. Además de las complicaciones relacionadas con
la enfermedad, existen efectos adversos graves debidos al tratamiento
antituberculoso (Anti-TB). Palabras clave: Tuberculosis. Pacientes hospitalizados. Sistemas de comunicación de reacciones adversas. Toxicidad de medicamentos. Irán. |
Kheirollah GHOLAMI, PharmD. Associate Professor& Chair Dept.
of Clinical Pharmacy. Faculty of pharmacy. Tehran University of Medical Sciences.
Tehran, Iran
Elahe KAMALI. PharmD. Iranian Adverse Drug Reaction Monitoring Center Undersecretary
of Food and Drug Affairs. Ministry of Health. Tehran, Iran.
Mahboubeh HAJIABDOLBAGHI, MD. Associate Professor & Chair, Imam Khomeini
hospital, Infectious disease department. Tehran University of Medical Sciences.
Tehran, Iran.
Gloria SHALVIRI. PharmD. MPH, Iranian Adverse Drug Reaction Monitoring Center
Undersecretary of Food and Drug Affairs.Ministry of Health. Tehran, Iran
INTRODUCTION
Tuberculosis has been one of the common diseases in human communities during the past 40 years. It has been reported by World Health Organization (WHO) that one third of the worlds population is infected with Mycobacterium tuberculosis resulting in 8.4 million new tuberculosis cases in 1999.1 This high incidence of infection has caused a large number of morbidity and mortality which is partly due to serious adverse reactions induced by Anti-TB drugs.2,3
The frequency and nature of Anti-TB induced ADRs have been the matter of concern in many communities. One of the serious ADRs detected in these studies is hepatotoxicity. There are differences in reported rate of hepatotoxicity induced by Anti-TB drugs in different studies.4-6 This reaction could be affected by the genotype of patients receiving these drugs e.g. rapid-acetylator patients are more susceptible for isoniazid induced hepatotoxicity. Studies show that the risk of hepatotoxicity in patients from India is higher than those reported in West (11.5% versus 4.3%).7 Regarding the difference reported between Asian and Western people in developing Anti-TB induced hepatotoxicity, it is necessary to detect the rate of Anti-TB induced ADRs with emphasize on hepatotoxic reactions in Iranian patients, since it could be helpful to revise the therapeutic protocols.
To the best of our knowledge this is the first study for assessing Anti-TB induced ADRs in Iranian patients.
METHODS
This descriptive study was conducted in an infectious disease department of a 1200 bed teaching hospital, from July 2001 to July 2002. All patients diagnosed with pulmonary TB entered the study. These patients routinely received combination of four anti-TB drugs (Isoniazid, Rifampin, Pyrazinamide and Ethambutol). Hepatitis was defined as increased liver enzymes more than five times the base line accompanied with clinical symptoms including jaundice, nausea, vomiting, abdominal pain and anorexia. Patients with chronic hepatic illnesses such as cirrhosis, chronic hepatitis and acute viral hepatitis were excluded from the study. Patients demographics, disease history, drug history and final diagnosis were recorded. All the patients who received anti-TB drugs were monitored for adverse drug reactions. The ADR definition used in this study is that of the WHO Any noxious or unintended response to a drug, which occurs at doses normally used in human for the prophylaxis, diagnosis or treatment of disease or for the modification of physiological function.8
Detection and Monitoring was done by interviewing patients, reviewing laboratory tests and medical charts. Consulting with physicians about the patients clinical problems and recorded ADRs was done routinely. A yellow card, the Form used for ADR reporting to national Pharmacovigilance Center, was filled for each ADR detected in the study.
To asses the probability, severity and preventability of the reactions, Naranjo algorithm9, Hartwig questionnaire10 and Shumock questionnaire11 were used respectively.
RESULTS
During the study period 83 patients were diagnosed with positive TB. These patients were put on routine treatment protocol. Of these patients, 44 (53%) developed at least one adverse drug reaction. Total number of 81 adverse reactions detected in this study. The 44 patients with ADR consisted of 23 females and 21 males.
Occurrence of adverse reactions led to prolongation in hospital stay for 26 (59%) patients. The rate of adverse reactions was various in different age groups. It does appear that with Anti-TB drugs used in this study the rate of ADRs increases with increased age (Table 1)
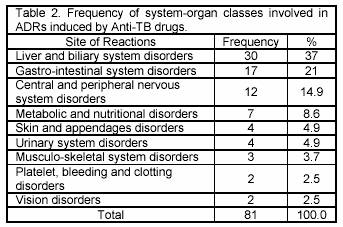
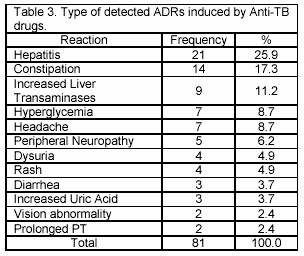
The most frequent system-organ classes affected by ADRs were Liver and biliary system (37%) and gastrointestinal system (21%) (Table 2). The most serious adverse reaction was hepatitis (25.9%), leading to death in two patients (Table 3). In this study, 30 (36.1%) patients have shown the raise in liver transaminases. However only 3 patients developed increased enzymes level less than 3 times of the base line. Furthermore these patients didnt show any clinical or biochemical evidence of hepatotoxicity. Increasing in plasma uric acid was observed in 3 patients (3.7%) due to Pyrazinamid. These reactions occurred in average 26.7 days after the beginning of treatment and followed by arthritis. After discontinuing of Pyrazinamid, the level of uric acid returned to normal range (2.1-8.5 mg/dl) in 10 days. Isoniazid caused reactions such as constipation (17.3%) and peripheral neuropathy (6.2%), while Rifampin was the major cause of headache (8.7%), rash and pruritus (4.9%) and diarrhoea (3.7%).
The only adverse reaction suspected to be induced by Ethambutol was vision abnormality such as blurred vision and burning eyes observed in two patients (2.4%).
The main action taken in patients with detected ADR was discontinuation of drug regimen (34.5%). This action mainly was taken when hepatotoxicity and/or hyperuricemia were detected. In 21% of patients with detected ADR, Anti-TB drug regimen was continued with symptomatic therapy. In 7.4% of the cases a decrease in Anti-TB drug regimen was performed. There was no specific treatment for alleviating the adverse reactions in 33.4% of cases. These reactions mainly were those which didnt cause a serious problem for patients, such as headache or constipation.
The outcome of 71.6% of adverse reactions in this study was definite improvement. Anti-TB drugs were discontinued after observing adverse reaction in 34.5% of patients. In 21% of reactions symptomatic treatment was performed.
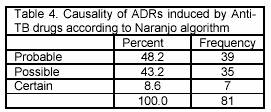
The causality assessment of ADRs revealed that 7 (8.6) cases were detected as certain, 35 (43.2%) as possible and 39 (48.2%) as probable reactions (Table 4).
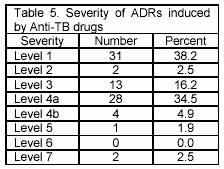
Evaluation of the severity of ADRs indicated that most of the ADRs detected had severity in level 1 (38.2%) and 4a (34.5%). (Table 5)
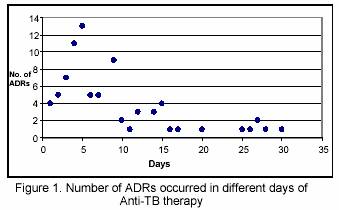
No adverse effect of anti-TB drugs were detected in the first 24 hours of drug therapy. The majority of adverse reactions (71.6%) were detected in the first 10 days of drug therapy (Figure 1). The frequency of adverse reactions in days 10-20 of drug therapy decreased to 19%. In days 21-30 the rate of ADRs detected was 21.4%. The adverse reactions that caused hospitalization mainly occurred on days 21-30 of drug usage.
DISCUSSION
Among 83 patients entered the study, 44 (53%) patients showed at least one adverse reaction. This relatively high percentage of occurring adverse reactions indicates that there is a need for more evaluation of susceptibility of Iranian patients for developing Anti-TB induced ADRs. Daphne Yee et al. conducted a study to estimate the incidence and risk factors of major side effects from first-line anti-TB drugs.12 They evaluated 430 patients treated with Anti-TB drugs between 1990 and 1999. The results of the study showed that the incidence of all major adverse effects was 1.48 per 100 person-months of exposure (95% confidence interval [95% CI], 1.31 to 1.61) for Pyrazinamide, compared with 0.49 (95% CI, 0.42 to 0.55) for isoniazid, 0.43 (95% CI, 0.37 to 0.49) for Rifampin, and 0.07 (95% CI, 0.04 to 0.10) for Ethambutol. The occurrence of any major side effect in that study was associated with female sex (adjusted hazard ratio, 2.5; 95% CI, 1.3 to 4.7), age over 60 years (adjusted hazard ratio, 2.9; 95% CI, 1.3 to 6.3), birthplace in Asia (adjusted hazard ratio, 2.5; 95% CI, 1.3 to 5.0), and human immunodeficiency viruspositive status (adjusted hazard ratio, 3.8; 95% CI, 1.05 to 13.4). Among these risk factors age over 60 years and birth place in Asia may be considered as interactive factors in inducing high rate of ADRs in our study. The results of this study as well as previous reports show that with increase of age, the frequency of ADRs will be increased.
In our study the major cause of admission was adverse drug reactions in 13.6% of patients. In similar study conducted in Iranian population hospitalized in general ward, ADR has been reported as the cause of admission for 8% of patients.13 In another study conducted for detecting Anti-Infectives induced adverse reactions in Iranian hospitalized patients, the total rate of hospitalization because of an ADR was estimated as 2.2%.14 These results suggest that Anti-TB drugs may cause more serious ADRs resulting in hospitalization comparing with other drug classes used in infectious and general wards which were subject of two other studies.
Hepatitis was observed in 21 patients (25.9%), leading to death of 2 patients. It has been estimated that 10%-20% of INH recipients develop elevated liver enzymes.15 However, In case of mild, subclinical hepatic damage, the reaction do not progress to overt hepatitis and recover completely despite of continuing INH therapy. In contrast, if clinical symptoms occur, severe hepatocellular toxicity could be happened which is associated with a higher fatality rate than that of patients whose INH was discontinued immediately. The exact mechanisms responsible for INH hepatotoxicity are still unclear. Slow or fast acetylation of INH in the liver has been the matter of controversy as a probable mechanism for INH hepatotoxicity. Previously, it was believed that rapid acetylators might be at a greater risk for INH hepatitis than slow acetylators. Monoacetylhydrazine, the hepatotoxic metabolite of Isoniazid, is formed more rapidly in fast acetylators comparing with slow acetylators. In contrast, rapid acetylators also would eliminate this compound at a faster rate, and this should equalize the risk of toxicity between slow and fast acetylators. One study demonstrated a different incidence of hepatitis between Asian males and females. Because both groups were fast acetylators, this study suggested that hepatitis is probably caused by factors other than acetylators phenotype. Thus it does appear that acetylators status alone does not explain the development of INH hepatitis. However, the results of this study suggest that more studies are needed to evaluate the risk of increased rate of INH induced hepatitis among Asian people.
Some evidence initially suggested that concurrent use of INH and Rifampin might lead to a greater incidence of hepatotoxicity. It is believed that Rifampin can induce the metabolism of INH to hepatotoxins. A meta-analysis conducted by Steele et al. looking at the incidence of hepatitis in all studies between 1966 and 1989 using regimens contained INH without Rifampin, Rifampin without INH and regimens containing both drugs, revealed that the incidence of clinical hepatitis was greater in regimens containing both INH and Rifampin (2.7%) versus regimens of INH alone (1.6%). The authors suggested that this effect was additive, not synergistic, therefore the use of the two drugs together, is not contraindicated. However, caution should be used in high-risk groups such as the elderly, alcoholics, those taking additional hepatotoxic agents, and those with pre-existing liver disease. If transaminase serum levels rise by using Anti-TB drugs, normal serum levels usually will be achieved after three to six weeks from the beginning of therapy. On time diagnosis of liver toxicity caused by Anti-TB drugs will reduce the rate of mortality of patients. The death rate due to liver damage will be about 50% if the drug is continued after increasing transaminase enzymes up to 3 times of base line. But with on time discontinuation of drug regimen, this rate could be decreased to 10 %.16
Increase serum level of transaminases mainly is due to Isoniazid. In the other hand Rifampin usually causes cholestasis, which leads to raise alkaline-phosphatase and bilirubin. Liver toxicities can be the major side effect of all three main anti-TB drugs, Isoniazid, Rifampin and Pyrazinamid.
Generally, in order to decrease the risk of liver damage of these drugs the
following points can be helpful:
Recording liver enzymes baseline levels before prescribing the Anti-TB drugs
Monitoring the transaminase serum levels in patients over 20 years of age.
This monitoring should be performed twice a week in the first two weeks and
once a week in the next two months.
Discontinuing the drug regimen immediately after raising the enzymes 3 times
of the baseline.
Avoiding concurrent use of Anti-TB drugs with CYTP450 inducers.
Avoiding concurrent use of Anti-TB drugs with other hepatotoxic medications.
CONCLUSION
In conclusion, Anti-TB drugs could cause significant adverse effects both in quantity and severity. These reactions may lead to hospitalization, prolonged hospital stay and even death. Asian People may develop more frequently severe adverse reactions, such as hepatitis, induced by this class of medicines. This result suggests that the protocol of Anti-TB therapy for Asian population may need some revision to prevent fatal hepatotoxicity. To confirm this hypothesis many more studies with larger population is needed.
|
References |
1. World Health Organization. Global Tuberculosis Control. WHO report 2001. Geneva, Switzerland: WHO/CDS/TB; 2001. 287. [ Links ]
2. Kopanoff DE, Snider DE, Caras GJ. Isoniazid-related hepatitis. Am Rev Respir Dis 1978:117:991-1001. [ Links ]
3. Burman WJ. Reves RR.. Hepatotoxicity from Rifampin plus Pyrazinamide. Lessons for Policymakers and Messages for Care Providers. Am J Respir Crit Care Med 2001; 164: 1112-3. [ Links ]
4. British Thoracic and Tuberculosis Association: Short course chemotherapy in pulmonary tuberculosis. Lancet 1975; 119-24. [ Links ]
5. Tanaja DP, Kaur D. Study on hepatotoxicity and other side effects of antituberculosis drugs. J Indian Med Assoc 1990; 88:278-80. [ Links ]
6. Snider DE, Long MW, Cross FS, Farer LS. Six months Isoniazid and Rifampin therapy for pulmonary tuberculosis: report of a United States Public Health Service cooperative trial. Am Rev Respir Dis 1984; 77:233-42. [ Links ]
7. Sharma SK., Balamurgan A., Saha PK., Pandey RM. Mehra NK. Evaluation of clinical and immunogenetic risk factors for the development of hepatotoxicity during Antituberculosis treatment. Am J Respir Crit Care Med. 2002;166:916-9. [ Links ]
8. World Health Organization. Uppsala Monitoring Center. Safety monitoring of medicinal products, guidelines for setting up and running pharmacovigilance center, Geneva, 1996. [ Links ]
9. Naranjo CA., Busto U., Sellers EM. A method for estimating the probability of Adverse Drug Reactions. Clin Pharmacother 1981; 30:239-45. [ Links ]
10. Hartwig SC., Siegel J., Schneider PJ. Preventability and severity assessment in reporting Adverse Drug Reactions. Am J Hosp Pharm, 1992; 49:2229-32. [ Links ]
11. Schumock GT, Thornton JP. Focusing on the preventability of Adverse Drug Reactions. Hosp Pharm. 1992; 27:538. [ Links ]
12. Yee D, Valiquette C, Pelletier M, Parisien I, Rocher I, Menzies D. Incidence of Serious Side Effects from First-Line Antituberculosis Drugs among Patients Treated for Active Tuberculosis. Am J Resp Crit Care Med. 2003. 167: 1472-7. [ Links ]
13. Gholami K., Shalviri G. Factors associated with preventability, predictability and severity of ADRs. Ann Pharmacother 1999; 33:236-40. [ Links ]
14. Gholami K, Parsa S, Shalviri G, Sharifzadeh M, Assasi N. Anti-infectives-induced adverse drug reactions in hospitalized patients. Pharmacoepidemiol Drug Safe 2005; 14:501-6. [ Links ]
15. Kays MB. Tuberculosis. In: Koda-Kimble MA, Young LY, Kradijan WA, Guglielmo JB, Allfredge BK, Corelli RL. Applied Therapeutics, The Clinical Use of Drugs. Eighth Edition 2005. Lippincott Williams and Wilkins. p.71-83. [ Links ]











 texto em
texto em