Meu SciELO
Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Pharmacy Practice (Granada)
versão On-line ISSN 1886-3655versão impressa ISSN 1885-642X
Pharmacy Pract (Granada) vol.5 no.3 Redondela Jul./Set. 2007
| Original Research |
Analysis of medicine advertisement produced in Brazil
Lorilei de Fátima WZOREK, Cassyano J. CORRER, Angela C. BADARÓ TRINDADE, Roberto PONTAROLO
| ABSTRACT Objective: To analyze the compliance of drug advertisements with regulations in Brazil, subject to Resolution RDC No. 102/2000 since 2000, which abides by the WHO's (World Health Organization) Ethical Criteria for Medicinal Drug Promotion, published in 1988. Key words: Advertising. Periodicals. Drug Industry. Brazil. | RESUMEN Objetivo: Analizar el cumplimiento de los reglamentos de publicidad de medicamentos en Brasil, sujetos a la Resolución RDC No. 102/2000 desde el 2000, que desarrolla los Criterios Éticos para la Promoción de Medicamentos de la OMS (Organización Mundial del a Salud) publicados en 1988. Palabras clave: Publicidad. Revistas. Industria farmacéutica. Brasil. |
Lorilei de Fátima WZOREK, BSc(Pharm). Pharmacy Practice Research Group, Universidade Federal do Paraná, Curitiba, PR (Brazil).
Cassyano J. CORRER, MSc. Pharmacy Practice Research Group, Universidade Federal do Paraná, Curitiba, PR (Brazil).
Angela C. BADARÓ TRINDADE, PhD. Pharmacy Practice Research Group, Universidade Federal do Paraná, Curitiba, PR (Brazil).
Roberto PONTAROLO. PhD. Pharmacy Practice Research Group, Universidade Federal do Paraná, Curitiba, PR (Brazil).
INTRODUCTION
All over the world, the interactions between drugs and public health are frequent objects of study for governments, universities, and non-governmental organizations. These interactions create a plethora of issues ranging from equal drug access, self-medication, intoxication, public access to information, and the influences of drug advertising. Several of these issues have also been addressed in the Health Ministry's National Drugs Policy guidelines.1
Specific studies on prescription drug advertising in Brazil between 1995 and 1999 showed2-5 that, on average, 72% of drug advertisements did not contain information on contraindications, side effects, adverse reactions, interactions, precautions and warnings.
The need for regulation was already a topic for discussion by the World Health Organization (WHO), which published the Criteria for Medicinal Drug Promotion in 1988 and recommended that member countries adopt control measures to ensure the public health.6 In Brazil, only on November 30th, 2000 did the Agência Nacional de Vigilância Sanitária (National Health Surveillance Agency - ANVISA) finally pass a board resolution, RCD No. 102/00, regulating prescription drug advertising.7
However, the mere existence of specific regulations is not a guarantee of their enforcement and compliance, as demonstrated by several research studies conducted after the publication of RDC No. 102/00.8-12 These studies confirmed that though 70% of Brazilian advertisements divulged the required essential information (contraindications, Common Brazilian and International Nomenclature (DCB/DCI), and adverse reactions), they still contained infringements of the regulations. These violations posed major health risks, such as the induction to indiscriminate use, suggestion of low risk, manipulation of scientific data to favor a given product, advertisement of nonregistered products, use of banned phrases and expressions that have an effect on prescription, prescription filling, and the proper use of advertised prescription drugs.
METHODS
We first selected the advertising media to be monitored; these included radio and television stations (at their regional program times), printed newspapers and magazines, and locations, including hospitals, medical clinics, conferences, health field events, drugstores, billboards, and bus doors. All media was monitored in Curitiba, PR and the metropolitan region. Prescription and prescription-free drug advertisements running from October 2002 to October 2003 were analyzed according to a modified version of the method proposed by Dal Pizzol, Silva and Schenkel4, Santi2, Silva5, Petrovick12, Carvalho8, Nascimento10, and Mastroianni.9 Three samples of each type of media and several locations were selected. Radio and television were monitored at least one hour per day at various times; Magazines and newspapers were monitored daily. Locations were visited every 15 days, with priority given to physicians' offices, drugstores, and places where advertising material aimed at the general public was on display. The material collected was classified as printed originals, billboard photographs and negatives, bus doors, and the audio and video recordings of radio and television advertisements, respectively. The objective of sampling was to survey advertisements and the type of products advertised. The media types and locations were changed periodically to obtain varied and new advertisements.
All advertised prescription drugs and products that should have been registered as medicines were analyzed in accordance to RDC No. 102/00 and the literature. The analysis looked for regulation violations according to a conformity checklist based on RDC No. 102/00 legal requirements. All material analyzed was submitted to ANVISA, the Brazilian health agency in charge of drug advertisement regulation and surveillance, to be checked for meeting legal requirements.
RESULTS
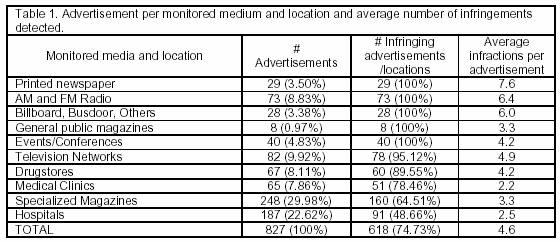
A total of 827 advertisements for 517 products were collected and analyzed. The regulation compliance analysis revealed that only 83.91% of the products (438 products of 694 advertisements) were regularly registered as drugs. Prescription-free drugs totalled 296 advertisements (42.65%), while prescription drugs totalled 340 advertisements (48.99%), and 58 drugs (8.38%) were subject to special control. Advertisements of unregistered products corresponded to 16.09% of the sample. Most (82.71%) advertisements announced non-registered phytotherapic products.
Of the sampled advertisements, 74.73% showed some regulation non-compliance. Non-prescription drug and prescription drug advertisements exhibited irregularities in 97.56% and 64.33% of the cases, respectively. Brazilian regulations permit open advertisement (running in television, newspapers, and magazines) of prescription-free drugs targeted to the general public; however, prescription drug advertisement is restricted to health-professional-targeted media.
Data on media distribution, location, and the number of infringing advertisements are shown in Table 1.
DISCUSSION
Radio stations, printed newspapers, and general public magazine advertisements showed the most infringements: 100% of the advertisements of these types analyzed had some form of infringement. These media vehicles reach nearly the entire Brazilian population and advertise prescription-free drugs. The advertisements attribute superior properties to the products announced, which exposes the population to health risk.
Many of the infringements detected suggest that advertisers intend to add value to the product by emphasizing supposed benefits and minimizing risks; such advertisements fail to mention the main contraindication (54.18%), minimize potential risk (23.91%), or an absence of side effects, and claim to be a "natural product" (19.30%).
In non-prescription drug advertisements specifically, the purchase and use of infringing drugs is stimulated in many ways: the association of the product with physical, emotional, or intellectual performance and/or personal beauty (32.43%), the suggestion of diagnose and treatment (31.75%), the stimulation of indiscriminate use (17.23%), the neglect of the mandatory warning "if symptoms persist, a doctor must be consulted," the offer of purchase-associated prizes (12.16%), and the claims of drug safety without side effects (11.83%). Furthermore, 21.28% of the advertisements do not provide required items, such as the registration number, the name of active ingredients, or a drug's commercial name.
In the case of prescription drugs, the infringements highlight the commercial character of advertising, which emphasizes benefits while it downplays risks, and suggests that the product is as risk-free as prescription-free drugs. In addition, these infringements grossly lack warnings and information on care (28.89%), contraindications (25.37%), dosage (24.37%), drug classification (16.55%), and indications (9.29%).
The requirement of divulging certain information, (e.g. dosage, contraindications, indications, warnings and care) in drug advertisements may seem unnecessary, since such products are targeted to doctors and other health professionals. However, in practice, such information is necessary, as revealed by research carried out in Brazil. Interviews with doctors, residents, and sixth-year Medicine students showed that 27% of the interviewees are updated through vendors` and laboratory information; furthermore, 73% of these health care workers acknowledged prescribing drugs without knowing their composition.
For a long time, drugs have been the main focus of the disease-health relationship in pharmaceutical advertisements, in which they are synonymous with being healthy - the message suggested that there is a medicine to prevent or to treat anything.
CONCLUSION
The drug advertisement samples analyzed showed the following conclusions: 1) 74.73% of the advertisements analyzed infringe currently enforced health regulations; 2) the main characteristic of advertisements is an over-emphasis on alleged benefits of drugs at the expense of risk and care information and warnings which might restrict prescription and inadequate use; 3) only 26% of advertisements completely infringed on regulations, showing that it is possible to to sell/advertise drugs in compliance with regulations; 4) the data suggest that most pharmaceutical companies disregard health regulations in force and adopt advertising campaigns that are inappropriate from legal and ethical perspectives, 5) our research suggests that the RDC No. 102/00 regulation has done little to improve drug advertising in Brazil; and finally, 6) our data reveal the need both to intensify surveillance and punish offender, as well as to promote health through educational campaigns aimed at health professionals and the general public.
ACKNOWLEDGEMENT
The authors appreciates the cooperation of the researchers and students who participated in the drug advertisement monitoring project, thanks the Agência Nacional de Vigilância Sanitária (Anvisa) for supporting this project and the American Journal Experts for review the manuscript.
CONFLICT OF INTEREST
None declared.
| References |
1. Ministério da Saúde. Política Nacional de Medicamentos. Brasília: MS; 2001. [ Links ]
2. de Santi V. Medicamentos: verso e reverso da propaganda. Ponta Grossa: UEPG; 1999. [ Links ]
3. Heineck I, Gallina SM, Silva T, Pizzo FD, Schenkel EP. Analise da publicidade de medicamentos veiculada em emissoras de radio do Rio Grande do Sul, Brasil. Cad Saude Publica. 1998;14(1):193-8. [ Links ]
4. Pizzol FD, Silva T, Schenkel EP. Analise da adequacao das propagandas de medicamentos dirigidas a categoria medica distribuidas no sul do Brasil. Cad Saude Publica. 1998;14(1):85-91. [ Links ]
5. Silva EV, Hoefler R, Moraes LB. Avaliação das propagandas de medicamentos distribuídas para a classe médica de Brasília. Farmacoterapêutica. 1999;IV(4):1-3. [ Links ]
6. Organización Mundial de la Salud. Critérios éticos para la promoción de medicamentos. Geneve: OMS; 1988. [ Links ]
7. Agencia Nacional de Vigilância Sanitária (ANVISA). RDC nº 102/00. Aprova o Regulamento sobre propagandas, mensagens publicitarias e promocionais e outras práticas(...). 2000 Dez 01 (cited 2004 Mar 01); Available from: www.anvisa.gov.br [ Links ]
8. Carvalho ACB, Fernandes MG, Santos EJV, Melo AFM, Medeiros IA, Diniz MFFM. Avaliação legal da propaganda e publicidade de medicamentos fitoterápicos anunciados na Paraíba (Brasil). Acta Farmaceutica Bonarense. 2004;23(3):413-7. [ Links ]
9. Mastroianni PC, Galduroz JC, Carlini EA. Influence of the legislation on the advertisement of psychoactive medications in Brazil. Rev Bras Psiquiatr 2003;25(3):146-55. [ Links ]
10. Nascimento AC. A persistirem os sintomas o médico deverá ser consultado: isto é regulação? São Paulo: Sobravime; 2005. [ Links ]
11. Resener MC. Análise da qualidade de propagandas de medicamentos fitoterápicos disponibilizadas em Santa Catarina (MSc Thesis). Florianópolis: Universidade Federal do Paraná; 2004. [ Links ]
12. Petrovick PR, Lima MA. Avaliação da Publicidade Visual de Medicamentos em Estabelecimentos Farmacêuticos de Porto Alegre-RS. Infarma (Revista Pharmacia Brasileira). 2003;15(1/3):52-6. [ Links ]
13. Costa EA. Vigilância Sanitária a Defesa e Proteção da Saúde (PhD Thesis). São Paulo: Universidade de São Paulo; 2004. [ Links ]