Meu SciELO
Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Pharmacy Practice (Granada)
versão On-line ISSN 1886-3655versão impressa ISSN 1885-642X
Pharmacy Pract (Granada) vol.6 no.4 Redondela Out./Dez. 2008
| Original Research |
The PharmWatch programme: challenges to engaging the community pharmacists in Jamaica
Maxine GOSSELL-WILLIAMS, Sarafadeen A. ADEBAYO
| ABSTRACT In February 2006, there was a renewed effort to encourage reporting of adverse drug reactions (ADRs) in Jamaica. It involved renaming the process the PharmWatch programme and revising the reporting form. Key words: Adverse Drug Reaction Reporting Systems. Pharmacists. Jamaica. | RESUMEN En febrero de 2006 hubo un nuevo esfuerzo para animar a la comunicación de reacciones adversas (RAM) en Jamaica. Conllevó el cambio de nombre a PharmWatch y la revisión del formulario de comunicación. Palabras clave: Sistemas de comunicación de reacciones adversas. Farmacéuticos. Jamaica. |
Maxine GOSSELL-WILLIAMS. PhD (Pharmacology). Department of Basic Medical Sciences, Pharmacology Section, University of the West Indies. Kingston (Jamaica).
Sarafadeen A. ADEBAYO. PhD (Pharmaceutics). School of Pharmacy and Health Science. University of Technology, Kingston (Jamaica).
INTRODUCTION
While clinical trials involving drugs provide significant information on the efficacy of pharmaceuticals, they provide limited information on the safety of their use because of the controlled conditions under which pre-marketing clinical trials take place. These trials occur with small numbers, sometimes excluding the elderly, children, patients with co-morbidities or on multi-drug therapy and are also of limited duration.1 Therefore there are inherent risks when drugs enter the general market and their usage essentially becomes a balancing act of benefits verses adverse drug reactions.
An adverse drug reaction (ADR), as defined by the World Health Organization2, is the response to a drug which is noxious and unintended and which occurs at doses normally used for the prophylaxis, diagnosis or therapy of a disease, or for the modification of physiological functions. These ADRs may be previously described or appear with use of the drug in the general market. To ensure that an ADR is not missed, it is estimated that at least 30,000 persons need to be treated with a drug to produce an incidence of 1 in 10,000 exposed persons.3 Therefore, adequate assessment of the safety profile of a drug requires active pharmacovigilance.
Pharmacovigilance, as defined by the World Health Organisation, is the pharmacological science and activities relating to the detection, assessment, understanding and prevention of adverse effects, particularly short- term and long -term side effects of medicines, or any other drug related problems.4 Therefore pharmacovigilance will effectively encourage safe, rational and more effective use of drugs. It has been estimated that through pharmacovigilance, 1 in every 5 drugs on the market has been discovered to have a serious ADRs (e.g. requiring hospitalization or causing death) resulting in changes made to drug monographs or withdrawal from the market.5 It was through pharmacovigilance that incidence of cardiovascular complications occurring with Cisapride, Terfenadine, Tegaserod and Rofecoxib warranted their removal from the market.6-8
In Jamaica, through the Pharmaceutical and Regulatory Unit of the Ministry of Health, all healthcare professionals are encourage to report ADRs using the spontaneous ADR monitoring form. This unit collects these reports, undertakes further investigations and takes appropriate actions. Oral communication with the Ministry of Health indicated that there is very little use of the spontaneous ADR form by health professionals. In 2006, through a collaborative effort involving the Ministry of Health and the Pharmacology Section of the University of the West Indies, there was a renewed effort to encourage utilization of spontaneous ADR reporting with the design of the PharmWatch programme. The original ADR reporting form was revised in February 2006 to produce a PharmWatch form to report ADRs. This form is currently available to all healthcare professionals and patients from the Ministry of Health and Pharmacology section of UWI.
The form was also placed on the website of the Jamaica Pharmaceutical Society in an effort to encourage pharmacists to become actively involved in the reporting process. Many countries have the active participation of pharmacists, especially community pharmacists in ADR reporting and have indicated that pharmacists involvement in this process can make significant contributions to the benefit verse risk analysis among populations.9-12 Therefore it is important to engage the Jamaican pharmacists in this process.
In January 2007, an islandwide survey of practicing community pharmacists was done. The aims of the survey were to assess the attitudes of community pharmacists to ADR reporting and their awareness of the PharmWatch programme.
METHODS
The study design employed a retrospective opinion survey of community pharmacists in Jamaica. The list of registered pharmacies as of August 2006 was obtained from the Pharmacy Council of Jamaica. One hundred and two pharmacies were randomly selected from the three counties of Jamaica (Cornwall, Middlesex and Surrey) to conduct the survey. The survey required one pharmacist from each pharmacy to complete a short questionnaire that was designed by nine pharmacists, each with over five years of community pharmacy practice experience. It was then validated by pharmacists in the pharmacy training programme at the University of Technology, Jamaica (UTech). The final questionnaire was drafted, taking reviewers comments into consideration. The questionnaire included demographic information, as well as their opinions on the importance of ADR reporting and whether they were aware of the PharmWatch programme. They were also asked to indicate the factors that affect their reporting of ADRs and to record all the ADRs they could recall occurring in the last twelve months. They were then provided with copies of the PharmWatch form, which they were required to use to record all ADRs occurring in the next three months (prospectively). Pharmacists were called on a weekly basis as a way to encourage the participation.
The information on the questionnaires was treated with the SPSS version 12 for frequency analysis.
RESULTS
Pharmacists (one per pharmacy) from 102 community pharmacies islandwide (Cornwall = 36, Middlesex = 36 and Surrey =30) participated in the survey; this represents 28.8% (total number of registered pharmacists = 354) of the pharmacies registered with Ministry of Health, as of August 2006. The majority, 75%, of the pharmacists had more than five years of professional experience in pharmacy practice.
Awareness of PharmWatch programme and challenges
The majority of the pharmacists involved in the survey, 67%, were not aware of the PharmWatch programme. However, 86 % of the responding pharmacists indicated that they accepted that ADR reporting was a professional responsibility and only 5% thought ADR reporting was a low priority task.
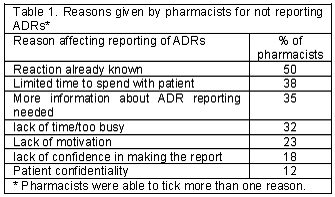
When pharmacists were asked what would contribute to them not reporting problems to the Ministry of Health, the top three reasons given were, reaction already known, more information needed about ADR reporting and lack of time (Table 1). However fifty percent of them indicated that reporting ADRs would increase if an incentive for reporting was granted.
Retrospective and prospective ADR reports
The questionnaire asked pharmacists to list any ADRs they could recall in the past twelve months. Over the three counties, 128 ADRs reports were collected of which none were reported to the Ministry of Health; but, two were reported to the respective pharmaceutical companies.
Once pharmacists agreed to participate in the survey and made aware of the PharmWatch programme, they were then asked to participate in a monthly follow-up for three months. During this time they were expected to record any ADRs occurring using the PharmWatch form. For this time period, 45 forms were collected, but in most cases only the name of the drug, the reaction that occurred and the action taken were noted. For both the retrospective and prospective ADRs, anti-infective drugs dominated the reports (39.1% and 37.8% of the reports respectively).
DISCUSSION
Pharmacovigilance on a national level is important for assessing the occurrence of differences that can result from factors related to diet, genetics, herbal product use or cultural practices.3 In this survey, we found that while community pharmacists were aware that they have a critical role in ADR reporting, most were not aware of the PharmWatch programme. Pharmacists involved in the survey were able to list 128 ADRs that occurred in the twelve months prior to participating in the survey; of which none were ever reported to the Ministry of Health. Reporting of ADRs plays a significant role in ensuring the safe use of drugs6; therefore the unavailability of reports to the Ministry of Health will affect the success of the PharmWatch programme. For example, the most common ADRs reported in this survey were associated with anti-infective drugs; while this is an expected trend13,14, their benefits are significantly influence by resistance development and therefore evaluation of these ADRs may be critical for resistance detection.
Another factor that requires community pharmacists active involvement in ADR reporting is the need to facilitate confidence in the efficacy of drugs. In the United States, through active surveillance of ADRs, it was determined that failure of therapy rated as the top ADR outcome during the period 1969 to 2002.6 Promoting ADR reporting to ensure efficacy is of particular importance in the Jamaican setting, where pharmacists are mandated to offer patients generic alternatives to the more expensive innovator brands. Most pharmacists (and physicians) have expressed lack of confidence in many generics15,16; however, with non-reporting of ADRs, the Ministry of Health would be unable to adequately address this issue.
Community pharmacists in this survey indicated reaction already known, lack of information about ADR reporting and confidence in making reports, as reasons for not reporting ADRs. Therefore, insufficient knowledge is a major challenge to community pharmacists participation in ADR reporting. Lack of time and motivation were also given as predominant reasons for non-reporting. These major challenges of Jamaican community pharmacists are consistent with those identified by pharmacists in other countries1,9,17,18, and suggests that pharmacists awareness of the PharmWatch programme is inadequate to promote the current pharmacovigilance system. This fact was very clear from quality of the prospective ADR reports collected; while this part of the survey netted 45 reports, in most cases, only the name of the drug, the reaction that occurred and the action taken were noted. Continuous training and encouragement in the use of the PharmWatch programme should therefore be considered; as such interventions can improve pharmacists participation in ADR reporting.19-21
CONCLUSION
Spontaneous ADR monitoring is a standard process proven to be an important part of evaluating the safety of drugs in the general market. This is the first report that assesses the attitudes of community pharmacists in Jamaica towards pharmacovigilance and specifically their willingness to participate in ADR reporting. While it is encouraging that most of the pharmacists were aware that they have a significant role in ADR reporting, much needs to be done to engage their participation including providing training opportunities.
ACKNOWLEDGEMENTS
This survey was made possible through funding provided by HD Hopwood and Company Limited, Medi-Grace Limited and Cari-Med Limited. The survey was conducted by practising pharmacists enrolled the Post-Diploma Pharmacy programme at the University of Technology.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
| References |
1. Zolezzi M, Parsotam N. Adverse drug reaction reporting in New Zealand:implications for pharmacists. Ther Clin Risk Manag. 2005;1(3):181-188. [ Links ]
2. World Health Organization. Safety of Medicines. Guidelines for setting up and running a pharmacovigilance centre. Geneva:WHO;2000. [ Links ]
3. World Health Organization. Safety of Medicines. A guide to detecting and reporting adverse drug reactions. Why health professionals need to take action. Geneva:WHO;2002. [ Links ]
4. Edwards IR , Aronson JK. Adverse drug reaction: Definitions, diagnosis and management. Lancet. 2000;356:1255-1259. [ Links ]
5. Lasser KE, Allen PD, Woolhandler SJ Himmelstein DU, Wolfe SM. Timing of new blackbox warnings and withdrawals for prescription medication. JAMA 2002;287:2215-2220. [ Links ]
6. Wysowski D, Swartz L. Adverse Drug Surveillance and Drug Withdrawals in the United States, 1969-2000. Arch Intern Med. 2005;165:1363-1369. [ Links ]
7. Rainsford KD. Anti-inflammatory drugs in the 21st century. Subcell Biochem 2007;42:3-27. [ Links ]
8. Sanger GJ, Alpers DH. Development of drugs for gastrointestinal motor disorders: translating science to clinical need. Neurogastroenterol Motil. 2008;20(3):177-184. [ Links ]
9. van Grootheest K, Olsson S, Couper M, de Jong-van den Berg L. Pharmacists role in reporting adverse drug reactions in an international perspective. Pharmacoepidemiol Drug Saf. 2004;13:457-464. [ Links ]
10. Terceros Y, Chahine-Chaktoura C, Malinowski JE, Rickley WF. Impact of a pharmacy resident on hospital length of stay and drug-related costs. Ann Pharmacother. 2007;41(5):742-748. [ Links ]
11. Phansalkar S, Hoffman JM, Nebeker JR, Hurdle JF. Pharmacists versus nonpharmacists in adverse drug event detection: a meta-analysis and systematic review. Am J Health Syst Pharm. 2007;64(8):842-849. [ Links ]
12. Viktil KK, Blix HS. The impact of clinical pharmacists on drug-related problems and clinical outcomes. Basic Clin Pharmacol Toxicol. 2008;102(3):275-280. [ Links ]
13. Bigby M. Rates of cutaneous reactions to drugs. Arch Dermatol. 2001;137(6):765-770. [ Links ]
14. Svensson CK, Cowen EW, Gaspari AA. Cutaneous drug reactions. Pharmacol Rev. 2001;53(3):357-379. [ Links ]
15. Gossell-Williams M. Generic Substitutions: A 2005 survey of the acceptance and perceptions of physicians in Jamaica. West Indian Med J. 2007;56(5):458-463. [ Links ]
16. Gossell-Williams M, Harriott K. Generic substitution in Jamaica: challenges to improving effectiveness. WHO Drug Information. 2007;21(4):294-299. [ Links ]
17. Green CF, Mottram DR, Rowe PH, Pirmohamed M. Attitudes and knowledge of hospital pharmacists to adverse drug reaction reporting. Br J Clin Pharmacol. 2001;51(1):81-86. [ Links ]
18. Irujo M, Beitia G, Bes-Rastrollo M, Figueiras A, Hernández-Díaz S, Lasheras B. Factors that influence under-reporting of suspected adverse drug reactions among community pharmacists in a Spanish region. Drug Saf. 2007;30(11):1073-1082. [ Links ]
19. Bracchi R, Houghton J, Woods F, Thomas S Small SA, Routledge P. A distant-learning programme in pharmacovigilance linked to educational credits is associated with imporved reporting of suspected adverse drug reactions via the UK yellow card system. Br J Clin Pharmacol. 2005;60(2):221-223. [ Links ]
20. Sweis D, Wong ICK. A survey on factors that could affect adverse drug reaction reporting according to hospital pharmacists in Great Britain. Drug Saf. 2000;23:165-172. [ Links ]
21. Herdeiro MT, Polonia J, Gestal-Otero JJ, Figueiras A. Improving the reporting of adverse drug reactions : a cluster-randomized trial among pharmacists in Portugal. Drug Saf. 2008; 31(4):335-344. [ Links ]