Meu SciELO
Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Pharmacy Practice (Granada)
versão On-line ISSN 1886-3655versão impressa ISSN 1885-642X
Pharmacy Pract (Granada) vol.8 no.2 Redondela Abr./Jun. 2010
ORIGINAL RESEARCH
Pharmacists´ participation in the documentation of medication history in a developing setting: An exploratory assessment with new criteria
Participación de los farmacéuticos en la documentación del historial de medicación en un país en desarrollo: evaluación exploratoria con nuevos criterios
Kazeem B. Yusuff1, Fola Tayo2, Bola A. Aina3
1. Kazeem B. YUSUFF. B.Pharm., M.Pharm., Ph.D. Senior lecturer. Department of Clinical Pharmacy & Pharmacy Administration. Faculty of Pharmacy, University of Ibadan. Ibadan (Nigeria).
2. B.Pharm, PhD. Professor. Department of Clinical Pharmacy & Biopharmacy. Faculty of Pharmacy, Idi-Araba Campus, University of Lagos. Lagos (Nigeria).
3. B.Pharm, MSc., PhD. Senior Lecturer. Department of Clinical Pharmacy & Biopharmacy. Faculty of Pharmacy, Idi-Araba Campus, University of Lagos. Lagos (Nigeria).
SUMMARY
Objective: To assess the impact of pharmacists´ participation on the frequency and depth of medication history information documented in a developing setting like Nigeria.
Method: The study consisted of two phases. The first phase was a baseline cross-sectional assessment of the frequency and depth of medication history information documented by physicians in case notes of systematic samples of 900 patients that were stratified over 9 Medical outpatients Units at a premier teaching hospital in south western Nigeria. The second phase was an exploratory study involving 10 pharmacists who conducted cross-sectional medication history interview for 324 randomly selected patients.
Results: 49.2% of patients, whose medication history were documented at the baseline, by physicians, were males; while 50.3% of patient interviewed by pharmacists were male. Mean age (SD) of males and females whose medication histories were documented by physicians and pharmacists were 43.2 (SD=18.6), 43.1 (SD=17.9) years and 51.5 (SD=17.6), 52.1 (SD=17.4) years respectively. The frequency of medication history information documented by pharmacists was significantly higher for twelve of the thirteen medication history components (P < 0.0001). These include prescription medicines; over the counter medicines; source of medicines; adverse drug reactions; allergy to drugs, allergy to foods, allergy to chemicals; patient adherence; alcohol use; cigarette smoking; dietary restrictions and herbal medicine use. The depth of medication history information acquired and documented by pharmacist was significantly better for all the thirteen medication history components (P<0.0001).
Conclusion: Pharmacists´ participation resulted in significant increase in frequency and depth of medication history information documented in a developing setting like Nigeria. The new medication history evaluation criteria proved useful in assessing the impact of pharmacists´ participation.
Key words: Medical Records. Pharmacists. Nigeria.
RESUMEN
Objetivo: Evaluar el impacto de la participación de los farmacéuticos en la frecuencia y profundidad de la información registrada en los historiales de medicación en un país en desarrollo como Nigeria.
Métodos: El estudio consistió en dos fases: la primera fase fue una evaluación basal transversal de la frecuencia y profundidad de la información registrada en los historiales de medicación por los médicos como casos de muestras sistemáticas de 900 pacientes que se estratificaron de 9 unidades ambulatorias en un hospital universitario en el suroeste de Nigeria. La segunda fase fue un estudio exploratorio que envolvió a 10 farmacéuticos que realizaron entrevistas transversales de historias de medicación a 324 pacientes aleatoriamente seleccionados.
Resultados: El 49,2% de los pacientes, cuyo historial de medicación fue documentado en el inicio por los médicos eran mujeres; mientras que el 50,3% de los entrevistados por los farmacéuticos eran hombres. La media (DE) de edad de los hombres y las mujeres con historiales de medicación registrados por los médicos y farmacéuticos era de 43,2 (DE=18,6), 43,1 (DE=17,9) años y 51,5 (DE=17,6), 52,1 (DE=17,4) años, respectivamente. La frecuencia de información registrada en los historiales de medicación por los farmacéuticos fue significativamente más alta para 12 de los 13 componentes (P<0,0001). Estos incluían los medicamentos prescritos; los medicamentos OTC; la fuentes de medicamentos; las reacciones adversas; la alergia a medicamentos, alimentos o substancias químicas; el cumplimiento del paciente; la ingesta de alcohol; el tabaco; las restricciones dietéticas y el uso de plantas medicinales. La profundidad de la información de los historiales de medicación adquirida y documentada por los farmacéuticos era significativamente mejor para todos los 13 componentes de los historiales (p<0,0001).
Conclusión: La participación de los farmacéuticos produjo un incremento significativo en frecuencia y profundidad de la información registrada en los historiales de medicación en un país en desarrollo como Nigeria. Los nuevos criterios de evaluación de historiales de medicación probaron ser útiles para evaluar el impacto de la participación de los farmacéuticos.
Palabras clave: Historias clínicas. Farmacéuticos. Nigeria.
Introduction
A medication history is a detailed, accurate and complete account of all prescribed and non-prescribed medications that a patient had taken or is currently taking prior to a newly initiated institutionalized or ambulatory care. It provides valuable insights into patients´ allergic tendencies, adherence to pharmacological and non-pharmacological treatments, social drug use and probable self-medication with complementary and alternative medicines.1,2 Availability of a detailed medication history, particularly at the prescribing stage of the medication use process, is critical to the success of the diagnostic and patient management tasks. This is because the occurrence of medication errors is strongly associated with inadequate or incomplete medication history.3-5
The documentation of medication history has historically been undertaken mainly by physicians and sometimes nurses. However, several studies which were done mainly in developed setting showed that the medication history information documented by physicians are often inaccurate, incomplete; and lack information regarding medication allergies, past prescription / non-prescription medications and patients´ adherence to prescribed doses.6-8 Furthermore, Yusuff and Awotunde9, in the only published study on the documentation of medication history by physicians in a developing setting like Nigeria, reported that the frequency of documentation was very low for hospitalized patients.
The participation of pharmacists in the documentation of medication history has been shown to result in significant improvement in its accuracy and comprehensiveness.10 Pharmacists-acquired medication histories are often free of error of commission, omission and more frequently document past prescription / OTC medicines, allergy history and use of alcohol.3,11-15 However, this concept has been tested only in developed countries.16-18 There is no published information on impact of pharmacists´ participation in the documentation of medication history in developing countries. This is notwithstanding that Nigeria, like other developing countries, is beset with poorly organized drug distribution system, poorly controlled access to both orthodox and herbal medicines, distribution / sale of counterfeit medicines, and pervasive self medication with prescription-only, over-the-counter and herbal medicines.19-25
Given the scenario described above, it is important that detailed accounts of all medications that patients had taken or are currently taking are available before initiating a new institutionalized or ambulatory medication use. This will ensure that physicians have access to critical medication history information which may be useful to the diagnostic and patient management tasks. In addition, an insight into perspectives from the developing world concerning medication history research beyond the developed countries is warranted; particularly because new evaluation criteria were developed and used for assessing the depth of the medication history information documented.
The objective of this study was to assess, for the first time in a developing setting like Nigeria, the impact of pharmacists´ participation on the frequency and depth of medication history information documented in an ambulatory tertiary care hospital.
Methods
The study was carried out at the University College Hospital (UCH), Ibadan. This is a 900-bed Teaching Hospital with medical residents located in Ibadan, Southwestern Nigeria. It is affiliated with the University of Ibadan. UCH is a teaching / tertiary hospital and a major referral center in Nigeria. It is also a major site for undergraduate and post-graduate residency training of physicians. Ethical clearance and approval of the study protocols was granted by the Joint University of Ibadan / University College Hospital Research and Ethics Committee. The study consisted of two phases, phase 1 (Baseline) and 2 (Exploratory).
Phase 1: Baseline Study
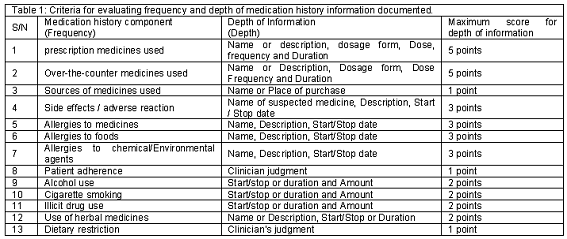
A retrospective cross-sectional study was carried out at the Medical Outpatient Clinics of University College Hospital (UCH), Ibadan between February and May, 2004. The case notes of 1803 patients that attended the Cardiology, Chest, Dermatology, Endocrine, Gastroenterology, Hematology, Neurology, Psychiatry and Renal Clinics in 4 weeks were included; and stratified across the nine medical units. Systematic samples of 100 case notes were selected from each of the nine strata. This was done by numbering the case notes sequentially within each stratum, and drawing every odd-numbered case note randomly until a sample of 100 case notes was made. Data extraction from the 900 case notes was carried out with the aid of two pre-tested data collection forms. The first form collected data such as patient´s hospital number, age and gender. The second form was a medication history evaluation criteria, which was designed to assess the frequency and depth of medication history information documented by physicians during clerking at patients´ first clinic visit (Table 1). The evaluation criteria were developed following extensive review of literature concerning medication history.1-2,15,26-27 Face and content validity of the evaluation criteria was assured through in-depth discussion with 2 Faculty senior colleagues, and 2 physicians at the Clinical Pharmacology Unit at the study site. The criteria were assessed for clarity and comprehensiveness during the pre-test. Pre-testing was done on forty-five randomly selected case notes at the General Outpatient Department in UCH. Modification of the medication history evaluation criteria was based on results of the pre-test. The frequency of documentation of each of the medication history component by physicians in all the nine specialties was assessed with these criteria. The depth of medication history information documented was assessed with a point system which assigns proportional scores based on the counts of medication history information documented divided by the maximum obtainable information points. This was done by first calculating the raw scores for each of the 13 medication history component. These raw scores were then divided by the maximum obtainable scores listed in the third column of Table 1. Hence, the proportional scores have a minimum-maximum range of 0 to 1.
For example, for prescription drug in a case note: Tab Metformin 500mg t.d.s 1/12.
Information contained includes (i) Tab; (ii) Metformin; (iii) 500mg; (iv) t.d.s; (v) 1/12.
Raw scores = 5 points;
Proportional scores = information contained ÷ maximum obtainable information;
Proportional score = 5/5 = 1.
Hence, proportional score for prescription drug in that case note is 1.
Documentation of "None" response or "No known drug allergy" was assigned the maximum proportional score (1) since such documentation infers that enquiries had been made. However, documentation of "Yes" or "No" for any of the medication history components was regarded as incomplete, and assigned a raw score of 1 point for at least presenting the information; since it was impossible to ascertain if enquiries were actually made. In addition, the absence of documentation for any of the medication history component was regarded as no documentation, and assigned a raw score of zero. However, it was impossible to ascertain the extent to which lack of documentation is indicative of non-enquiry. It is possible that some patients´ responses which were negative or "no response" were not considered necessary for recording.
Phase 2: Exploratory study
This was a cross sectional interviews of randomly selected 324 patients who presented at the Medical Outpatient Clinic, UCH over a four-week period (25th October - 19th November, 2004). All the patients who presented at the Medical Outpatient Clinics at the study site within the study period constituted the sampling frame. All patients attending the Psychiatric clinic, all severely ill and unconscious patients, and all patients who do not wish to take part in this phase were excluded. Patient selection was done after clinic appointment cards have been collected and patients were seated in the waiting area. Twenty clinic appointment cards were randomly drawn daily from the collected pool for the 4 weeks study period. The patients whose cards were drawn were called out by the Matron to the interview area. The informed consents of selected patients were obtained after the aim of the study had been explained to them.
The interviews were carried out by 10 pharmacists with a medication history data sheet which was developed following review of existing literature.1-2,15,26-27 The medication history data sheet was structured to ensure that bias due to difference in pharmacists´ interviewing skills or recall ability was minimized. The first draft of the medication history data sheet was pre-tested on 20 patients at the General Outpatient clinic at the study site. Modification of the final draft was based on result of the pre-testing.
The interviews were conducted daily between 8a.m and 10am with the medication history data sheet in separate consulting rooms. This ensured that all patients were interviewed before physicians´ consultation began. The pharmacists´ interviews were timed using a stopwatch. No patient was interviewed twice by pharmacists within the 4 weeks study period to avoid second-recall bias. All completed medication history data sheet were retrieved daily. The criteria (Table 1) used for evaluation of the frequency and depth of patient medication history documented by physicians at the baseline phase were also used to assess the frequency and depth of medication history information recorded by pharmacists.
Data analysis was carried out with Statistical Package for Social Sciences (SPSS) window version 10.0. (SPSS Inc., Chicago, IL) which report exact p-values. An a priori level of statistical significance of P< 0.05 was used for all comparison. Chi-square statistics and Independent t-test were used to assess the impact of pharmacists´ participation on the frequency and depth of medication history documented respectively. This was achieved by comparing the frequencies and mean scores of the depth of all the thirteen medication history components acquired by pharmacists with the frequencies and mean score of the depth of the thirteen medication history components documented in patients´ case notes by physicians.
Results
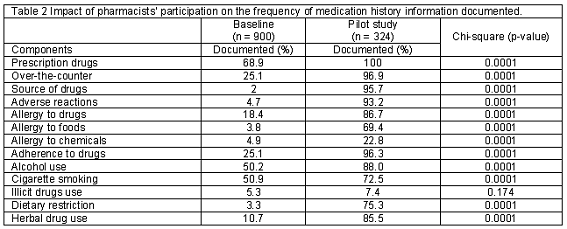
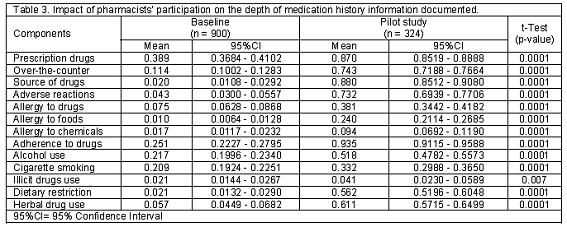
Of the patients interviewed by physicians (900), 49.2% (443) were males while 50.8% (457) were females. 50.3% (163) of the patient interviewed by pharmacists were male while 49.7% (161) were female. There was no significant difference in distribution across gender between the two groups (P>0.05). Furthermore, the mean age for patients at the baseline phase is 43.2 (SD=18.6) and 43.1 (SD=17.9) years for male and female respectively. While for the patients interviewed by pharmacists during the pilot study, the mean age for males and females were 51.5 (SD=17.6) and 52.1 (SD=17.4) years respectively. Patients interviewed by pharmacists were significantly older (P<0.0001). The mean time taken to interview patients by pharmacists at the exploratory study phase was 10.73 (SD=2.58) minutes (minimum=6.8 minutes, maximum=16.5 minutes). The comparison of the frequency of documentation of all the thirteen medication history components at the baseline and exploratory study phase is as shown in Table 2. The frequency of medication history information documented by pharmacists was significantly higher for twelve of the thirteen medication history components (P<0.0001). These include prescription medicines; over the counter medicines; source of medicines; adverse drug reactions; allergy to drugs, allergy to foods, allergy to chemicals; patient adherence; alcohol use; cigarette smoking; dietary restrictions and herbal medicine use. However, pharmacists´ participation did not significantly increase the frequency of documentation of patients´ use of illicit drugs (P>0.05). Table 3 shows the comparison of the depth of documentation of the thirteen medication history components documented at the baseline and pilot study phases. The depths of medication history information documented by pharmacists were significantly better for all the thirteen medication history components (P<0.0001).
Discussion
The frequency of documentation of prescription medicines patients had used increased from 68.9% to 100% following the participation of pharmacists. This finding is consistent with the results of Montpetit and Roy (68% to 91%)13 and Nester and Hale (38% to 61.8%)15. Our finding with regards to the increase in the documentation of OTC medicines previously used (25.1% to 96.9%) following pharmacists´ participation is similar to the results of Akwagyriam et al. (11.7% to 88.2%).17 Furthermore, our finding showing an increase in the documentation of past use of herbal remedies (10.7% to 85.5%) with pharmacists´ participation is also similar to the results of Nester and Hale15 who reported that pharmacist documented use of herbal remedies in 78.6% of patients compared to 21.4% recorded in case notes. The availability of detailed information on prior use of prescription, OTC and herbal medicines is potentially useful in identifying patients´ past inappropriate use of medicines, medication use-induced injuries.11
The frequency of documentation of sources of medicines patients had used or are currently using significantly increased from 2% to 95.7% following pharmacists´ participation. This information is particularly useful in Nigeria and other developing countries; where drug regulatory capacity is inadequate and sales of counterfeits and substandard medicines is still pervasive.20
Our study showed that the documentation of ADRs significantly increased from 4.7% to 93.2% following pharmacists´ participation; and this is consistent with the results of Akwagyriam et al. (26.1% to 73.9%).17 Furthermore, the documentation of allergies to drugs, foods and chemicals also increased significantly. This finding, which is in agreement with that of Massey and Bouwmeester et al, clearly underscored the critical role of pharmacists in preventing inadvertent prescription of medicines patients may be allergic to.12,28-30
Adherence to prescribed medicines is important for the attainment of positive patient outcomes; and non-awareness of adherence tendencies may lead to unwarranted dose increase or unnecessary addition of new medications.31 This underscores the importance of our finding which showed that the documentation of patients´ adherence to previous drug therapy significantly increased from 25.1% to 96.3% following pharmacists´ participation. This finding is consistent with the results of Badowski et al. who reported that pharmacist´ participation resulted in increased documentation of non-adherence with drug therapy in 95% of patients.32 However, pharmacists´ participation did not impact significantly on the documentation of prior use of illicit. This lack of difference appears due to the reported difficulty inherent in obtaining this medication history component, due probably to the societal stigma associated with the use of illicit drugs.2
Our findings concerning the increase in the depth of documentation of all the thirteen medication history components following pharmacists´ participation is consistent with the results of Montpetit & Roy, who reported that, following pharmacists´ participation, the mean scores for the depth of information on prescription and OTC medicines, side effects, allergies, adherence, alcohol use, cigarette smoking, and use of illicit drugs increased significantly.13
The mean time required to complete the medication history interview by pharmacists was 10.73 (SD=2.58) minutes (Minimum: 6.8 minutes, Maximum: 16.5 minutes). This finding is in agreement with those of Gleason et al., McRobbie et al. and Cradock et al. who all reported an average of 10 minutes.11,18,33 However, other authors have reported a slightly longer mean interviewing time (13 to 14 minutes).17,18 The average interviewing time by pharmacists appeared short and hence may not interfere with the routine care process. Furthermore, the devolution of the task of documenting medication history to pharmacists may relieve physicians to perform other important clinical tasks, thereby improving service delivery and fostering better inter-professional relationship.
The findings of this study should be interpreted in the light of the following limitations. It was impossible to assess the accuracy of the medication history information documented at both the baseline and pilot studies due to non-availability of an organized integrated system for collection, filing and accessing dispensing data from the community / hospital pharmacies and private medical practice in Nigeria. Furthermore, the absence of documentation of any of the medication history information by physicians at the baseline and by pharmacists during the exploratory study was regarded as no documentation and assigned a score of zero. However, it was impossible to ascertain the extent to which lack of documentation is indicative of non-enquiry. It is possible that some patients´ responses to medication history enquiries were not considered important or necessary for recording. Furthermore, pharmacists are currently not involved in routine documentation of medication history in Nigeria and other developing setting; hence, the extent to which pharmacists´ excitement with an opportunity to participate in documenting medication history at the exploratory phase interferes with the frequency and depth of information documented is not readily clear. However, the pharmacists were not aware that the frequency and depth of medication history information documented by them was compared with those documented in the case notes by physicians.
Conclusions
In conclusion, pharmacists´ participation resulted in significant increase in frequency and depth of medication history information documented. The medication history evaluation criteria proved useful in assessing the impact of pharmacists´ participation. Hence, the devolution of this task to pharmacists may be beneficial even in a developing setting like Nigeria.
Acknowledgement
To Mrs. B. Alao, Fateye Babasola, Prof. B. Salako and Dr. F. Fehintola; and the Master of Pharmacy students (02/03 set) for their contributions toward the successful conduct of the study.
Conflict of interest
We declare that there is no conflict of interest on this research study. This research study did not receive fund or support from any source.
References
1. Covington TR. Interviewing and advising patients. In Francke DE, Whitney HAK Jr. eds. Perspectives in Clinical Pharmacy, 1st edition, Illinois: Hamilton 1972. [ Links ]
2. Tietzie K. Clinical skills for pharmacists, a patient-focused approach. Mosby-year book inc. St Loius, Missouri. USA, 1997;39-40. [ Links ]
3. Cohen J, Wilson C, Ward F. Improve drug history taking. Pharmacy in Practice.1998;1:13-16. [ Links ]
4. Gandhi TK, Weingart SN, Borus J, Seger AC, Peterson J, Burdick E, Seger DL, Shu K, Federico F, Leape L, Bates DW. Adverse drug events in ambulatory care. N Engl J Med. 2003;348:1556-1564. [ Links ]
5. Wilcock M, Lawrence J. Is there a role for community pharmacists in identifying discrepancies in medication histories for patients admitted to hospital? Pharm J. 2004;272:253-256. [ Links ]
6. Beers MH, Munekata M, Storrie M. The accuracy of medication histories in the hospital medical records of elderly persons. J Am Geriatr Soc. 1990;38:1183-1187. [ Links ]
7. Batty GM, Oborne CA, Swift CG, Jackson SH. The use of over-the-counter medication by elderly medical inpatients. Postgrad Med J. 1997;73(865):720-722. [ Links ]
8. Bedell SE, Jabbour S, Goldberg R, Glaser H, Gobble S, Young-Xu Y, Graboys TB, Ravid S. Discrepancies in the use of medications: their extent and predictors in an outpatient practice. Arch Intern Med. 2000;160:2129-2134. [ Links ]
9. Yusuff KB, Awotunde M. The frequency of drug history documentation in an institutionalized tertiary care setting in Nigeria. J Pharm Pharm Sci. 2005; 8(2):140-145. [ Links ]
10. Tam VC, Knowles SR, Cornish PL, Fine N, Marchesano R, Etchells EE. Frequency, type and clinical importance of medication history errors at admission to hospital: a systematic review. Can Med Assoc J. 2005;173(5):510-515. [ Links ]
11. Gleason KM, Groszek JM, Sullivan C, Rooney D, Barnard C, Noskin GA. Reconciliation of discrepancies in medication histories and admission orders of newly hospitalized patients. Am J Health-Syst Pharm. 2004;61:1689-1695. [ Links ]
12. Massey C. An evaluation of the benefits of pharmacist-acquired drug histories. Proc Guild Hosp Pharm.1987;25:80-81. [ Links ]
13. Montpetit LM, Roy MT. Evaluation of a patient-completed versus health professional-conducted medication history. Drug Intell Clin Pharm. 1988;16:592-595. [ Links ]
14. Hocking G, Kalyanaraman R, deMello WF. Better drug history taking: an assessment of the drug mnemonic. J R Soc Med. 1998;91:305-306. [ Links ]
15. Nester TM, Hale LS. Effectiveness of a pharmacist-acquired medication history in promoting patient safety. Am J Health-Syst Pharm. 2002;59(22):2221-2225. [ Links ]
16. Gurwich EL. Comparison of medication histories acquired by pharmacists and physicians. Am J Hosp Pharm.1983;40:1541-1542. [ Links ]
17. Akwagyriam I, Goodyer LI, Harding L, Khakoo S, Millington H. Drug history taking and the identification of related problems in an accident and emergency department. J Accid Emerg Med. 1996;13:166-168. [ Links ]
18. McRobbie D, Bednall R, West T. Assessing the impact of re-engineering of pharmacy services to general medical wards. Pharm J. 2003;270:342-345. [ Links ]
19. Eniojukan JF, Aina BA, Tijani MM. Drug distribution network in Nigeria Nig Quart J Hosp Med. 1997;7:384-389. [ Links ]
20. Erhun WO, Babalola OO, Erhun MO. Drug regulation and control in Nigeria: The challenge of counterfeit drugs. J Health Pop Develop Countries. 2001;4(2):23-34. [ Links ]
21. Taylor RB, Sharkoor O, Behrens RH, Everard M, Low AS, Wangboonskul J, Reid RG, Kolawole JA. Pharmacopoeial quality of drugs supplied by Nigerian pharmacies. Lancet. 2001;357(9272):1933-1936. [ Links ]
22. Ositelu A. Ocular drug abuse in Lagos, Nigeria. Acta Opthalmologica 1989;67:396-400. [ Links ]
23. Erhun WO, Agunedu CO. Procurement and sale of drugs in a market-place. Nig J Pharm. 1995;26:30-32. [ Links ]
24. Ajayi IO, Falade CO. Pre-hospital treatment of febrile illness in children attending the General Outpatient Clinic, University College Hospital, Ibadan, Nigeria. Afr J Med Med Sci. 2006;35:85-91. [ Links ]
25. Dunne J. Deadly medicines: the cost of substandard drugs. Medicine Digest 1997;23:5-7. [ Links ]
26. Maponga C, Odari C. The quality of antimalarial; A study in selected African countries. World Health Organization. Geneva. WHO/EDM/PAR. 2003;4:1-54. [ Links ]
27. Alubo SA. Drugging the people: pills, profits and underdevelopment in Nigeria. Studies in Third World Societies. 1985;24:89-113. [ Links ]
28. Bouwmeester M, Laberge N, Bussieres J, Lebel D, Bailey B, Harel F. Program to remove incorrect allergy documentation in pediatrics medical records. Am J Health-Syst Pharm. 2001;58:1722-1727. [ Links ]
29. Geibig CB, Mansur JM, Medema PG. Pharmacy-controlled documentation of drug allergies. Am J Hosp Pharm. 1991;48:260-264. [ Links ]
30. Tripp DM, Brown GR. Pharmacist assessment of drug allergies. Am J Hosp Pharm. 1993;50:95-98. [ Links ]
31. Bieszk N. Detection of medication non-adherence through review of pharmacy claims data. Am J Health-Syst Pharm. 2003;60(4):360-366. [ Links ]
32. Badowski SA, Rosenbloom D, Dawson PH. Clinical importance of pharmacist-obtained medication histories using a validated questionnaire. Am J Hosp Pharm. 1984;41:731-732. [ Links ]
33. Cradock JC, Whitefield GR, Menzie JW. Post-admission drug allergy histories recorded by pharmacists. Am J Hosp Pharm. 1972;29:250-252. [ Links ]
Received: 13.01.10
Accepted: 19.05.10