INTRODUCTION
Parkinson’s disease (PD) is a chronic, irreversible neurodegenerative disorder, induced by massive dopamine loss and it is recognized by motor and non-motor symptoms.1PD available treatment is symptomatic with no proven efficacy to slow or reverse the disease progression.2The estimated prevalence of PD, in 2004, among Lebanese population is 0.36%.3Lebanese PD annual mortality rate is 0.2 per 100,000 people due to disease progression, and or medication related adverse events.4
Levodopa and dopamine agonists are considered essential options in early and advanced PD treatment guidelines.5,6Among all multiple antiparkinsonian therapies that are now available, the standardized levodopa equivalent dose (LED) is set to provide a useful tool to compare dose intensities of different medications in clinical trials. It is the dose that produces the same antiparkinsonian effect as 100 mg of immediate release levodopa.7
Pramipexole is a non-ergot dopamine agonist approved for the treatment of early PD. It is indicated either to delay the use of Levodopa, or as an add-on to Levodopa for patients with advanced PD experiencing Levodopa induced dyskinesia, and off periods.8,9Various in vivo and in vitro studies have showed that pramipexole has promising neuroprotective effects10,11, while clinical human studies did not.12Pramipexole was expected to hold a significant potential in PD treatment since it is associated with lower motor complication risks, and higher reduction in Unified Parkinson Disease Rating Score (UPDRS).13,14UPRDS is used to follow the longitudinal course of PD and is divided into 6 sections, where section III includes a clinician-scored motor evaluation that can be used as a measure of Pramipexole efficacy on motor functioning.14
Pramipexole is available in two formulations; an immediate release (IR), and a once-daily extended release (ER) formula. The ER was verified to be bioequivalent to the IR formulation.15Furthermore the ER formulation was associated with better patient adherence to therapy, constant plasma concentration drug level, improved clinical response, and safety profile.15,16Several studies investigated the effect of pramipexole addition to PD patients’ regimens on levodopa daily dose.17,18According to our knowledge there are no studies comparing the addition of pramipexole ER into the same patient’s regimen before and after Pramipexole ER introduction.
Moreover, there is not enough information regarding the PD treatment, complication, and prognosis among the Lebanese population. Therefore, this study was undertaken to quantify the potential benefit of pramipexole ER on total LED and UPDRS-III score. The primary objective was to compare the total LED before and after introduction of pramipexole ER into the regimen for each individual PD patient.
METHODS
Study Design
This is a retrospective observational, single arm study conducted in a specialized PD and movement disease center in Lebanon between January and December 2017. Initially, total of 698 subjects’ records were screened with PD. PD patients were excluded from the study if they had history of any psychiatric disorder, atypical PD symptoms, dementia, hypotension or any other clinical significant disease at any point of their lives, to make sure that the symptoms encountered are solely due to Parkinson disease. Patients were also excluded if they had received any of the following drugs; typical and atypical antipsychotics, centrally active antiemetic, amphetamine, and/or methyldopa because these medications have the ability to cause Parkinson-like side effects.
Only 176 PD patients who were shifted from their ongoing antiparkinsonian therapy (consisting of mono- or combined therapy of pramipexole IR and/or carbidopa/levodopa) to mono or combined pramipexole ER therapy were included in the study. The main reason for introducing pramipexole ER to the patients’ regimens was due to the lack of efficacy of the previous regimens.
It is worth noting that pramipexole dose was increased by 0.75 mg/day every week to reach a maximum of 4.5 mg/week, whereas the levodopa/carbidopa initial dose was 25 mg-100 mg orally three times a day or 10 mg-100 mg orally 3 or 4 times a day, increased by 1 tablet every day or every other day as needed, until a dose of 8 tablets (2000 mg) was reached. When adding pramipexole ER to the regimen, the doses were adjusted according to patients’ symptoms.19
The enrolled patients’ medications were recorded and their baseline total levodopa equivalent dose (LED) was calculated before and after shifting the patient to pramipexole ER. LED was calculated based on the conversion factors inTable 1.
Table 1. Levodopa equivalent daily dose conversion factors5
| Drug | Conversion factor |
|---|---|
| Levodopa | x 1 |
| Entacapone | LD x 0.33 |
| Tolcapone | LD x 0.5 |
| Pramipexole | x 100 |
| Ropinirole | x 20 |
| Rotigotine | x 30 |
| Selegiline oral | x 10 |
| Selegiline sublingual | x 80 |
| Rasagiline | x 100 |
| Amantadine | x 1 |
| Apomorphine | x 10 |
Outcome measures
The primary endpoint was defined as the change in LED after introducing Pramipexole ER into the regimen. The secondary endpoint was defined as the mean change from baseline to week 13 in the UPDRS-III score. A cutoff point for a minimal clinical important difference in UPDRS-III was a decrease in 2.5-5 points.20,21Other endpoints observed were the change in LED based on age (<65 vs. >65 years) and the severity of motor symptoms defined as mild for UPDRS-III <32, moderate 33-58, and severe >59.22
Data Analysis
Statistical analysis was performed using the SPSS version 20.0. Descriptive statistics were used to describe patient characteristics (frequencies and percentages for categorical variables), and mean (standard deviation) for continuous variables. Pearson chi-square test was used to study the association between different categorical variables and student-t-test for continuous variables. The LED before and after pramipexole ER introduction in the same patient was compared for statistically significant differences using the paired student-t-test. A decrease in LED was analyzed as a drug dose reduction and/or elimination from baseline PD regimens. All reported p-values were two-sided with the alpha set at a significance of 0.05.
RESULTS
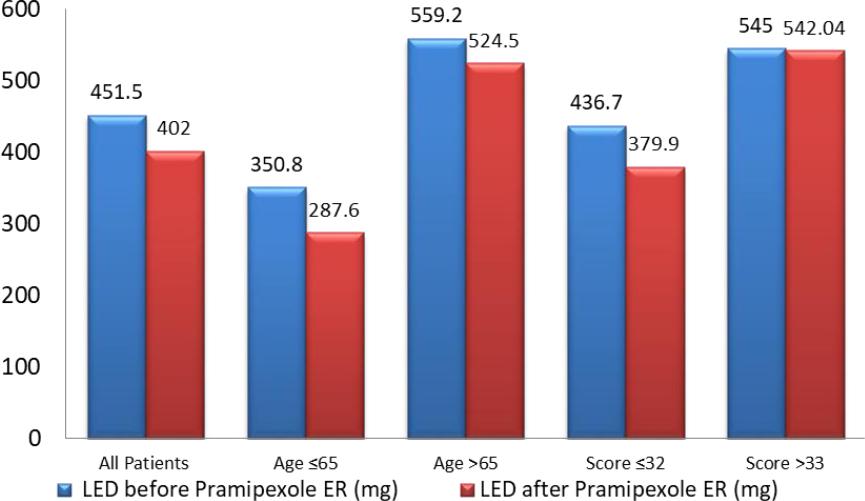
Out of 176 patients 54.5% were males and 45.5% were females. The mean age of the patients ranged from 27-88 was 60 years old (SD=8.1), 56.8% were less than or equal to 65 years. Regarding the disease duration, the mean was 2.5 years (SD=2.4). The mean baseline LED was 451.5 mg and the mean baseline UPDRS-III score was 18.99 points (SD=12.7) with 86.3% of patients less or equal to 30. (Table 2)
Table 2. Baseline patient characteristics
| Variable | N=176 |
|---|---|
| Age | Mean: 60.7 (SD=8.39) |
| < 65 | 100 (56.8) |
| > 65 | 76 (43.2) |
| Gender | |
| Male | 96 (54.5) |
| Female | 80 (45.5) |
| Duration of PD (y) | 2.5 (2.6) |
| UPDRS III | 18.9 (12.9) |
| Total Levodopa equivalent dose | 451.5 mg (272.5) |
Data are mean or number of subjects (%) unless otherwise indicated.
Pramipexole ER showed a significant difference on the mean changes in LED and UPDRS-III score. After 13 weeks of initiating Pramipexole ER, the mean decrease in LED was for all patients -49.42 mg (p<0.001, 95%CI [35.28-63.55]) and the mean decrease in UPDRS-III score for all patients was -6 points (p<0.001).
Subgroup analyses
In patients aged 65 years and below, the change in mean total LED from baseline (350.80 mg) was a decrease of 63.19 mg with a p<0.001, 95%CI [42.07-84.31]. In patients aged more than 65 years, the change in mean total LED from baseline (559.25 mg) was a decrease of 34.67 mg with a p<0.001 CI 95% [16.16-53.18].
In patients having an UPDRS-III score of less than or equal to 33, the change in mean total LED from baseline (436.73 mg) was a decrease of 56.76 mg (p<0.001; 95%CI [41.32-72.20]). However, in patients having an UPDRS-III score of more than 33 the change in mean total LED from baseline (545.06 mg) was a decrease of 2.96 mg with a p value<0.844 95%CI [27.32-33.15] (Figure 1).
DISCUSSION
According to our knowledge there are no studies evaluating the change in total LED in the same patient before and after introduction of pramipexole ER in Lebanon. In this study, it was observed that the total LED can be significantly reduced. Either due to symptom’s improvement or patient’s adherence using the Pramipexole ER, PD medication’s dose or agents were reduced or removed from the regimen. This result was consistent with other related studies.23,24
Our study showed an 8 points decrease in UPDRS-III score at 13 weeks with pramipexole ER, greater than the suggested cutoff point for efficacy. A similar study demonstrated a decrease of 6.1 points in pramipexole ER treated group.25
When taking age into consideration, pramipexole ER use was associated with lower LED in both groups (≤65 and >65 years), this indicates that pramipexole ER is an effective option in elderly patients. This correlates well with previous studies done to evaluate the efficacy of pramipexole in older patients.26 27 28-29A research that was conducted Pellicano et al. showed that pramipexole ER has levodopa sparing effect in patients older than 65 years 16. This theory is aligned with the standards of care in levodopa therapy among older adults with functional impairments due to the potential of worsening of the quality of life as well as greater risk of psychiatric side effects with dopamine agonists versus levodopa. However, in terms of doses, pramipexole ER decrease LED in older patients therefore further studies are required to proven the effect of dose reduction to be either beneficial or clinically insignificant and unnecessary.
With respect to the UPDRS-III score pramipexole ER was significantly correlated with lower LED total doses in patients with mild motor symptoms.
(UPDRS-III ≤33). In contrast, pramipexole ER levodopa sparing effect in patients having moderate to severe motor symptoms (UPDRS-III >33) was not significantly different. This result correlates well with other studies showing that the use of pramipexole ER was associated with lower LED in patients having mild-moderate PD30,31and not with severe PD, which can be explained by the need of combination therapies and higher doses to control advanced PD symptoms.
Although PD is a progressive degenerative disorder, pramipexole ER showed its ability to spare or delay the use of high levodopa doses and the absolute dependency to Levodopa, thus sparing the patient from complications that may arise from levodopa multiple daily dosing.
Limitations
Though our results were homogeneous with most previous studies, there still exist several limitations. Some of the limitations in this study are that it is retrospective and single-centered. Extra patients’ visits before week 13 were not recorded. Concerning efficacy measures, we used the UPDRS-III section only, while in other trials more parts of this scoring system were used. Besides, the LED conversion factors are not universally unified and thus may affect the accuracy and reliability of our results. We couldn’t study the prevalence of side effects before and after the addition of pramipexole ER and those of levodopa since data was not readily available in patient’s charts. In addition, adherence to the treatment along with baseline characteristics such as co-morbidities and treatment patterns couldn’t be retrieved from the available patients’ charts as well because of the retrospective nature of the study. Future studies that will address all these limitations are needed.
CONCLUSIONS
The total LED is significantly lowered when pramipexole ER is introduced to the same patient. The dose reduction is mostly prominent in patients aged less than 65 years with an UPDRS score less than 33. Furthermore, patients aged more than 65 years can also benefit from the LED reduction. This study opens new horizons on the potential role of health care professionals in maintaining the patient on the lowest effective levodopa equivalent daily dose and optimize the treatment therapy, thus decreasing the side effects that might arise from overdosing of antiparkinsonian drugs.