INTRODUCTION
Invasive infections due to Candida spp., generally referred to as invasive candidiasis, is recognized as a major cause of morbidity and mortality in hospitalized patients.1 According to the Centers for Disease Control and Prevention (CDC) it is estimated that approximately 46,000 cases of healthcare-associated candidiasis occur each year in the United States.2 In patients with invasive candidiasis, all-cause mortality rates range from 10-49.8% while attributable mortality has been reported in 10-20%.1,3,4 While there are at least 15 distinct Candida species that can cause human disease, >90% of invasive infections are caused by 5 pathogens: C. albicans, C. glabrata, C. tropicalis, C. parapsilosis, and C. krusei.2 Of these, C. krusei is inherently resistant to fluconazole and C. glabrata possesses low-level intrinsic resistance that may be overcome by increased doses of fluconazole.2 In contrast to previous trends in rates of candidemia increasing over 20 years, declines have been observed in the past 5-7 years.5 Despite this favorable trend, fluconazole-resistant Candida infections are becoming more prevalent.2
In December 2015, the Infectious Diseases Society of America (IDSA) published an updated clinical practice guideline for the management of candidiasis.1 Recommended management of candidemia includes initial (empiric) therapy with an intravenous echinocandin, followed by oral or intravenous fluconazole after azole susceptibility has been confirmed (approximately 5-7 days). Per IDSA, fluconazole is also an acceptable alternative to an echinocandin as initial therapy in select patients who are not critically ill and unlikely to have a fluconazole-resistant Candida species. The pharmacokinetics of fluconazole, a drug extensively eliminated by the kidneys but metabolized in the liver, are altered in patients with renal impairment and reduction in maintenance doses should be made according to established recommendations in previously published literature and prescribing information.6 In addition to drug treatment, patient- and disease-specific factors may impact treatment outcomes in patients with invasive candidiasis. Among these are the presence of immunosuppression, severe or life-threatening comorbidities, and the need for supportive therapies (such as mechanical ventilation and renal replacement therapy [RRT]).1 Prior studies have identified that the risk of death from candidemia is predicted by increasing age, higher severity of illness, immunosuppression, renal dysfunction, Candida spp., presence of central venous catheter, and inappropriate or delayed antifungal therapy.1,3,7,8 Factors positively affecting patient outcomes include early initiation of appropriate antifungal therapy and attainment of source control (i.e., removal of indwelling urinary or central venous catheters).1 A recent retrospective surveillance study of patients over 21 years of age with candidemia identified RRT as independent predictor of all-cause mortality.3 However, treatment outcome in these patients may have been impacted by treatment selection, and detailed information pertaining to the type and duration of concurrent RRT was not included. In addition, the study’s endpoint (all-cause mortality) may have been influenced by factors other than candidemia treatment response. Among these factors, the data regarding the impact of RRT on treatment outcomes in this patient population is especially sparse. Our purpose was to examine the impact of select risk factors, most notably concurrent RRT on treatment outcomes among adults with candidemia.
METHODS
This Institutional Review Board (IRB)-exempted, retrospective, case-cohort study was conducted at Duke University Hospital (DUH), a 938-bed academic medical center located in Durham, NC. Patients were identified and data were extracted utilizing the Duke Enterprise Data Unified Content Explorer (a web-based query tool providing direct access from Duke Medicine Enterprise Data Warehouse) and Maestro Care (DUH’s electronic medical record). Patients over 18 years of age admitted to DUH between June 1, 2013 and June 1, 2017 and had at least one positive blood culture for Candida spp., received a minimum of 3 doses of appropriate antifungal therapy (was defined as therapy concordant with IDSA guidelines for empiric and definitive treatment of invasive candidiasis) and had a follow-up encounter (defined as readmission or outpatient visit) over 7 days and within 90 days from completion of antifungal therapy were included. Patients were excluded if medical records were incomplete for the primary endpoint, received additional antifungal therapy for treatment of non-candidemia infection, and/or received subsequent prophylactic or suppressive antifungal therapy. Data collected included patient demographics, hospitalization (admission date/time, discharge date/time, status at discharge, intensive care unit (ICU) dates of stay and date(s) of follow up), fungal blood cultures (date, time, results and susceptibility), antifungal therapy (drug name, dose, frequency, and route of administration), description of RRT (method and dates identified via ICD-9 and ICD-10 codes), and absolute neutrophil count (ANC) at time of candidemia diagnosis.
The primary objective of this study was to compare the incidence of treatment failure among adult patients with candidemia and concurrent RRT. Secondary objectives were to compare the incidence of treatment failure among adult patients with candidemia and at least one of the following risk factors: treatment with fluconazole monotherapy, ICU stay, and neutropenia (ANC<500).
For all outcomes, treatment failure was defined as meeting any of the following criteria: 1) death prior to blood culture clearance (from any cause); 2) blood culture following index blood culture and within 14 days after completion of antifungal therapy positive for same Candida spp.; or 3) escalation of antifungal therapy secondary to clinical decompensation thought due to persistent or worsening fungal infection (i.e., persistent fever or hypotension). Cases absent of criteria meeting treatment failure definitions were categorized as treatment success.
An odds ratio (OR) and 95% confidence interval (95%CI) were calculated for the following during therapy: receipt of RRT, fluconazole monotherapy regimen, ICU stay at any point during treatment of candidemia, and neutropenia. Based on an alpha level of 0.05 and effect size of 20% a sample size of 76 would be required for 80% power, for the primary endpoint. For the primary endpoint, the use of concomitant RRT was compared between outcome groups using chi-square or Fisher’s exact test, as appropriate. Secondary endpoints were compared using Chi-square or Fisher’s exact test, as appropriate. For any characteristics(s) deemed to have a p-value≤0.20 between outcome groups, a multivariable logistic regression model would be used to identify association of those characteristics with treatment failure. All data were entered into Microsoft Access™, version 16.0.4549.1000 (Microsoft Corp., Redmond, WA). JMP©, version 13 (SAS Institute, Cary, NC), was used for all statistical calculations.
RESULTS
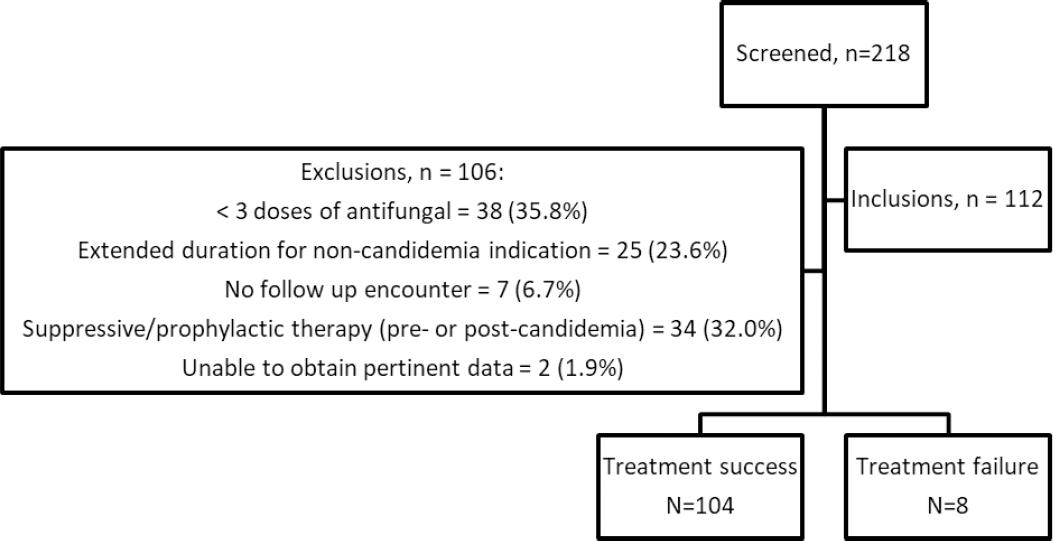
Of the 218 encounters screened for eligibility, 112 encounters (from 110 unique patients) were included (Figure 1). The most common reasons for exclusion were receipt of < 3 doses of an antifungal agent (n = 38), receipt of subsequent suppressive or prophylactic antifungal therapy (n = 34), and extended duration of antifungal therapy for non-candidemia indication (n = 25). Overall, patients evaluated were primarily male (57.1%), Caucasian (61.6%) or African American (33.0%), and ranged in age from 18.0 to 91.7 years of age (median 56 years) upon arrival to DUH. Treatment failure was observed in 8 (7.1%) of the 112 encounters. Baseline characteristics regarding race, sex, age at arrival, and discharge disposition were comparable between outcome groups (Table 1). C. albicans and C. glabrata were the two most common organisms, isolated in 36.5% (n=42) and 38.3% (n=44) of all patients, respectively. These were the only organisms isolated in patients with treatment failure (Table 2).
Table 1. Patient demographics among patients with candidemia by treatment outcome
| Parameter | Treatment success N = 104, n (%) | Treatment failure N = 8, n (%) | All encounters N = 112, n (%) |
|---|---|---|---|
| Age at arrival, years | |||
| Median (range) | 56.8 (1.7 – 91.7) | 52.8 (20.3 – 61.5) | 56.0 (18.0 – 91.7) |
| Male | 60 (57.7) | 4 (50.0) | 64 (57.1) |
| Race | |||
| Caucasian | 66 (63.4) | 3 (37.5) | 69 (61.6) |
| African American | 33 (31.7) | 4 (50.0) | 37 (33.0) |
| Other/not reported | 2 (2.0) | 1 (12.5) | 3 (2.7) |
| Multiracial | 2 (1.9) | 0 (0.0) | 2 (1.8) |
| American Indian or Alaskan Native | 1 (1.0) | 0 (0.0) | 1 (0.9) |
| Discharge disposition | |||
| Expired | 25 (24.0) | 2 (25.0) | 27 (24.1) |
| Home health service | 23 (22.1) | 3 (37.5) | 26 (23.2) |
| Home or self-care | 22 (21.2) | 1 (12.5) | 23 (20.5) |
| Skilled nursing facility | 14 (13.5) | 1 (12.5) | 15 (13.4) |
| Long term acute care | 8 (7.7) | 0 (0.0) | 8 (7.1) |
| Hospice (inpatient) | 6 (5.8) | 1 (12.5) | 7 (6.3) |
| Hospice (home) | 3 (2.9) | 0 (0.0) | 3 (2.7) |
| Federal hospital | 1 (0.9) | 0 (0.0) | 1 (0.9) |
| Left against medical advice | 1 (0.9) | 0 (0.0) | 1 (0.9) |
| Rehabilitation facility | 1 (0.9) | 0 (0.0) | 1 (0.9) |
| Characteristic of interest | |||
| Receipt of RRT | 11 (10.6) | 1 (12.5) | 12 (10.7) |
| Fluconazole monotherapy regimen | 66 (63.5) | 6 (75.0) | 72 (64.3) |
| ICU stay | 56 (53.8) | 5 (62.5) | 61 (54.5) |
| Neutropenia | 2 (1.9) | 0 (0.0) | 2 (1.8) |
RRT: renal replacement therapy; ICU: intensive care unit
Table 2. Causative organisms among patients with candidemia by treatment outcome
| Organism | Treatment Success, na (%) | Treatment Failure n (%) | All Encounters na (%) |
|---|---|---|---|
| C. glabrata | 41 (38.4) | 3 (37.5) | 44 (38.3) |
| C. albicans | 37 (34.6) | 5 (62.5) | 42 (36.5) |
| C. parapsilosis | 12 (11.2) | 0 (0.0) | 12 (10.4) |
| C. tropicalis | 12 (11.2) | 0 (0.0) | 12 (10.4) |
| C. dubliniensis | 2 (1.9) | 0 (0.0) | 2 (1.7) |
| C. krusei | 1 (0.9) | 0 (0.0) | 1 (0.9) |
| C. lusitaniae | 1 (0.9) | 0 (0.0) | 1 (0.9) |
| C. pelliculosa | 1 (0.9) | 0 (0.0) | 1 (0.9) |
a:3 patients had > 1 organism isolated
Among 12 patients receiving concomitant RRT, only 1 patient failed therapy. No significant differences were observed with regards to secondary outcomes related to treatment failure with a fluconazole monotherapy regimen (OR, 1.59; 95%CI, 0.3 - 8.27), ICU stay (OR, 1.43; 95%CI, 0.32 - 6.29), and neutropenia (0 treatment failures) (Table 3). No characteristic met the pre-specified p-value for performance of the multivariable logistic regression.
Table 3. Univariate analysis of risk of treatment failure among select subgroups in patients with candidemia
| Characteristic | Failure #failed/#exposed (%) | cOR | 95% CI |
|---|---|---|---|
| Renal replacement therapy | 1/12 (8.3) | 1.21 | 0.14 – 10.75 |
| Fluconazole monotherapy regimen | 6/72 (8.3) | 1.72 | 0.33 – 8.99 |
| ICU stay | 5/61 (8.2) | 1.43 | 0.32 – 6.29 |
| Neutropenia | 0/2 (0.0) | --- | --- |
ICU: intensive care unit; CI: Confidence Interval; cOR: Crude odds ratio
DISCUSSION
The overall treatment failure rate in our study (7.1%) was substantially lower than that reported in the published literature of 30 to 40%.1,3,9,10 Enrollment criteria requiring follow-up encounter over 7 days and within 90 days from completion of antifungal therapy may have significantly limited inclusion of patients with advanced malignancies or severe comorbidities. Patients were also excluded for receipt of subsequent antifungals as preventative or suppressive therapy. Fifty-nine of 106 (55.7%) encounters were excluded from our study due to meeting these criteria. These excluded patients may represent those at highest risk for treatment failure or relapse explaining their need for continuing therapy. Our study also required all patients to have received 3 or more doses of appropriate antifungal therapy prior to inclusion, thus resembling more of a “per protocol” analysis compared to other studies where no minimal treatment duration was specified.3 Our institution is a tertiary-care, academic medical center employing mandatory consultation after identification of a positive blood culture for yeast, the primary team is required to place an infectious diseases consult to aid in management of invasive candidiasis. Such consultation has been shown previously at our institution to improve adherence to quality of care indicators for such infections.11
In the present study, the rate of concomitant RRT in the overall study population (10.7%) was lower than the 12% to 33% described in previously published literature.3,9,10 Differences may be due to composite patient characteristic categories (i.e., “renal dysfunction”) including patients requiring hemodialysis, patients diagnosed with chronic renal failure based on serum creatinine, and patients with diagnosed chronic renal failure or non-specific categories (i.e., “renal insufficiency” or “renal failure”) with no further delineation. The current study relied on ICD-9 and -10 codes in addition to EMR records for receipt of RRT. Only 1 of the 12 patients receiving RRT failed therapy for candidemia. Reasons for differences in treatment outcomes likely parallel the reasons for differences between our study and others relative to overall success rates.
The impact of treatment selection on treatment outcome has been examined in prior studies.1,4,9,10 A patient-level review of randomized trials for treatment outcomes of invasive candidiasis found a significant association with receipt of an echinocandin pertaining to improved survival and greater clinical success.9 Two recent studies discuss the impact of initial antifungal strategy on outcomes of critically-ill patients after propensity-score matching was performed.10,11 One retrospective, propensity-score adjusted, analysis reported empirical use of an echinocandin was found to be a protective factor of 30- and 90-day mortality while de-escalation to fluconazole was not associated with a higher mortality.10 In contrast, a prospective, propensity-score derived analysis found targeted or empirical use of fluconazole was not associated with higher 30-day mortality compared to echinocandins among adult patients.12 Among the 72 patients in the present study whose treatment regimen contained fluconazole, 6/8 (75%) experienced treatment failure, and 6/6 (100%) of failures received fluconazole as initial therapy. In comparison, 66 among the 104 (63.5%) of patients receiving fluconazole as step-down therapy were successfully treated. Such observations are consistent with studies (and the basis for current treatment guidelines) which support the use of an echinocandin as initial empiric therapy followed by de-escalation to azole therapy when the patient is clinically stable and an azole-susceptible organism is identified.1,10
Neutropenia and severity of underlying illness may also impact treatment outcomes in patients with candidemia. In prior reports, the low numbers of patients with neutropenia (5 – 12% of study populations) has led to the inability to extrapolate results from retro- or prospective studies of outcomes in candidemia patients.7,10,11,12 In the present study, only 2 (1.7%) of the patients were neutropenic during treatment, both of whom were treated successfully. Severity of underlying illness also has been shown to influence treatment outcomes.1,3,7,9,10,12 All-cause mortality rates in critically ill patients with candidemia range from 18.7 % to 33.9%.3,10,11,12 ICU stay and increasing APACHE II, sepsis-related organ failure assessment (SOFA) scores on admission have both been demonstrated to be predictive of a higher risk of death in patients with invasive candidiasis.3,9,10,12 In the present study, of those patients requiring an ICU stay, 5/61 (8.2%) experienced treatment failure.
There are limitations to our study worth noting. The study was retrospective in nature, and therefore dependent upon the availability and accuracy of the medical records (most notably reliance on accuracy of ICD-9 and ICD-10 codes and administration of antifungal agents). Because of this, several factors which have been associated with treatment failure were not evaluated (e.g. timing of initiation of appropriate antifungal therapy and attainment of source control). While risk adjustment was not employed to create comparable outcome groups seen in some other studies, our high success rate was not likely to be impacted by such adjustments.10,12 Due to the specific nature of our patient population and characteristics studied, a small sample size is also a limitation of the study. Stringent inclusion criteria likely pre-screened for healthier patients to be evaluated leading to a limited number of patients requiring RRT and subsequent low rate of mortality. Lastly, dosages of fluconazole were difficult to assess for each patient as the dosage strategy was adjusted based on renal function, RRT flow rates and modality.
CONCLUSIONS
In the present study we were unable to detect an impact of RRT on treatment outcomes in adult patients with candidemia. This finding is likely impacted by the high treatment success rates reflective of a population receiving appropriate antifungal therapy and for whom adequate clinical and microbiologic data can be collected to more specifically measure impact of candidemia. No other characteristic investigated (receipt of fluconazole monotherapy regimen, ICU stay, and neutropenia) demonstrated statistical significance between outcome groups.