Meu SciELO
Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Revista de Osteoporosis y Metabolismo Mineral
versão On-line ISSN 2173-2345versão impressa ISSN 1889-836X
Rev Osteoporos Metab Miner vol.7 no.1 Madrid Jan./Mar. 2015
https://dx.doi.org/10.4321/S1889-836X2015000100002
A986S polymorphism of calcium-sensing receptor and osteoporotic clinical fractures
Polimorfismo A986S del receptor sensor del calcio y fracturas clínicas osteoporóticas
Briongos-Figuero L.S., Abad-Manteca L., Cuadrado-Medina F., Pineda-Alonso M., Vega-Tejedor G., Pérez-Castrillón J.L.
Servicio de Medicina Interna - Hospital Universitario del Río Hortega - Valladolid
SUMMARY
Introduction: The relationship between osteoporosis and arterial hypertension has not been clearly established, with alterations in calcium metabolism having been reported in the latter which may explain their association. Our objective was to establish the relationship between the A986S polymorphism of the calcium-sensing receptor (CaSR) and the presence of osteoporotic clinical fractures in a group of patients with hypertension.
Material: Prospective observational cohort study in 71 patients with hypertension, from 2001 to June 2014. We obtained socio-demographic and clinical data, including osteoporotic clinical fractures. The CaSR polymorphism was analysed using molecular techniques. The data was analysed using SPSS 15.0 (p<0.5)
Results: 43.77% of the patients were men and 56.3% women. Genotype AA was found in 67.6% of patients, genotype SS in 2.8% and genotype AS in 29.6%. Those with genotype AA did not have higher comorbidity (27% vs 26%, p=0.9) or more pathological fractures (14.6% vs 21.7%, p=0.4) than the others. In the subgroup of women, 11 osteoporotic clinical fractures were recorded, without there being any differences between those with the AA genotype and the others (28% vs 27%, p=0.9).
Conclusions: We found no association between the A986S polymorphism and the presence of osteoporotic clinical fractures in our cohort.
Key words: osteoporosis, hypertension, calcium-sensing receptor, risk of fracture.
RESUMEN
Introducción: La relación entre osteoporosis e hipertensión arterial no está claramente establecida, habiéndose descrito en ésta última alteraciones del metabolismo del calcio que podrían explicar su asociación. Nuestro objetivo fue establecer la relación entre el polimorfismo A986S del receptor sensor del calcio (CaSR) y la presencia de fracturas clínicas osteoporóticas en un grupo de pacientes hipertensos.
Material: Estudio de cohortes prospectivo observacional en 71 pacientes hipertensos, desde 2001 hasta junio de 2014. Obtuvimos datos sociodemográficos y clínicos, incluyendo fracturas osteoporóticas clínicas. El polimorfismo del CaSR se analizó con técnicas moleculares. Analizamos los datos con SPSS 15.0 (p<0,5).
Resultados: El 43,7% de los pacientes eran varones y el 56,3% mujeres. El genotipo AA se encontró en el 67,6%, el SS en el 2,8% y el AS en el 29,6%. Aquéllos con genotipo AA no presentaban más comorbilidad (27% vs. 26%, p=0,9) ni fracturas patológicas (14,6% vs. 21,7%, p=0,4) que el resto. En el subgrupo de mujeres se recogieron 11 fracturas osteoporóticas clínicas, sin encontrar diferencias entre el genotipo AA y el resto (28% vs. 27%, p=0,9).
Conclusiones: No hemos encontrado asociación entre el polimorfismo A986S y la presencia de fracturas osteoporóticas clínicas en nuestra cohorte.
Palabras clave: osteoporosis, hipertensión, receptor sensor del calcio, riesgo de fractura.
Introduction
Osteoporosis and arterial hypertension (AHT) are entities with a high prevalence in the general population, both clinically silent but becoming symptomatic when complications appear, such as fractures and cardiovascular diseases. Recent studies suggest that AHT is a risk factor for the appearance of pathological fractures [1,2]. Thus, in patients with hypertension various alterations in the metabolism of calcium have been described, such as an increase in ionic calcium and an increase in PTH and calciuria, although only this last change has been associated with an increase in bone mass [3-5].
The calcium sensor receptor (CaSR) is coupled with G proteins and detects the extracellular concentrations of calcium. It is expressed in various cell types (kidney, bone - both in the osteoblasts and osteoclasts - and brain), although it is in the parathyroid cells where there is the highest density. Thus, CaSR plays an essential role in the homeostasis of calcium, regulating the calcium-parathyroid hormone (PTH)-vitamin D axis. On the other hand, CaSR controls the excretion and reabsorption of calcium in the ascending limp of the loop of Henle, acting on different transporters and also intervening in the regulation of arterial pressure [6-9]. The polymorphism of codon 986 (A986) of CaSR is associated with an increase in blood levels of calcium and a decrease in calciuria, data antagonistic to those observed in arterial hypertension. Therefore, the objective of our study was to determine whether the presence of the S allele of the A986 polymorphism of CaSR might exert a protector effect on the appearance of clinical osteoporotic fractures in a group of patients with hypertension.
Material and methods
We designed a prospective observational study, initiated in 2001. A cohort of healthy people, with AHT as the only cardiovascular risk factor, was randomly selected with the aim of describing the incidence of complications over time. The patients were at stages I or II of systolic or diastolic hypertension, according to the criteria of the VI Meeting of the Joint National Committee (1997). The exclusion criteria were alcoholism, the presence of neoplasms, secondary hypertension, chronic renal insufficiency, hyper- or hypocalcaemia, diabetes, hyperparathyroidism and the use of drugs which may modify bone mineral density (BMD). The average duration of the hypertension was 7±8 years. A total of 71 patients were monitored until June 2014, taking at this point a cross section of our group to be studied. All the patients signed their informed consent and the study was approved by the clinical research committee of the Río Hortega University Hospital.
At the start of the study an analysis was made of calcium, phosphorus and magnesium using a Hitachi 917 autoanalyzer (Tokyo, Japan) and a densitometry of the lumbar spine (L2-L4) carried out with a Lunar densitometer (DEXA, Lunar Corporation, Madison, Wisconsin, US). Subsequently, socio-demographic and clinical data was obtained from the digital clinical records, collecting data regarding treatments with different antihypertensives, the development of concomitant diseases and the incidence of pathological osteoporotic fractures (distal radial, vertebral and femoral neck) over time. The comorbidity was assessed using the Charlson Index (CI) in its original version of 19 items, as has been described in the literature [10].
The analysis of the A986S polymorphism was performed using molecular biology techniques. The gene for CaSR is located in the 3q21.1 chromosome and can have inactivate or activate mutations. The A986S polymorphism is located in exon 7 and involves a change between alanine (A) and serine (S) in the intracellular C-terminal extreme of the receptor which generates a loss of function of the CaSR 9. To establish the genotype, a sample of blood anti-coagulated with EDTA was taken and the DNA extracted using the QIAmp Blood kit (Qiagen, Hilden, Germany). We designed the primers to amplify exon 7 (direct primer 5'CTTTGATGAGCCTCAGAAGAGC3' and inverse primer 5'ACAACTCTTCAGGGTCCTCC3'), and the direct primer was modified by introducing a base change, thus creating a palindromic sequence which allowed us to recognise the nucleotide changes using restriction enzymes. The PCR was carried out using 25mM of each dNTP (Applied Biosystems, Branchburg, New Jersey, U.S.), 50 mM of ClK, 10 mM of Tris HCl (ph 8.3), 1.5 U of the DNA polymerase Amplitaq (Applied Biosystems, Foster City, California, U.S.), 2.5 mM of Cl2Mg and 20 pmol of each primer, thus obtaining a total volume of 40 μl. The PCR was carried out in a Perkin Elmer 9600 thermal cycler (Norwalk, Connecticut, EE.UU.) with a temperature control system. The fragments were analysed by electrophoresis in an 8% acrylamide gel. Following the amplification the samples were added to the primers to create the specific restriction alleles for the BsaHI enzyme. The fragments obtained were digested by BsaHI (New England Biolabs, Stockholm, Sweden) and separated by electrophoresis in agarose gel. The presence of the BsaHI restriction fragment represents A, while its absence represents S, generating the genotypes AA, AS and SS.
The data were analysed with the statistical software package SPSS v15.0 (SPSS Inc®) with a level of significance for p≤0.05.
Results
Until the cross section of 2014 a total of 71 patients had been followed up, of whom 43.7% were men and 56.3% women, with an average age of 73.3±9 years, similar in both sexes (72.5±9 in the men and 74±9 in the women: p=0.5). 48% of our patients were more than 75 years of age. 17% of the patients died during the follow up period (all in the last two years).
At the baseline there were no differences between the two groups analysed (genotype AA and genotypes AS+SS). The rates of systolic (155±24 vs 154±20 mmHg, p=0.841) and diastolic (106±33 vs 93±11 mmHg, p=0.569) arterial pressure were similar. There were no differences in the levels of blood calcium (9.5±0.5 vs 9.8±0.4 mg/dl, p=0.098)or in calciuria (219±124 vs 275±175 mg/24h, p=0.268). The BMD in the lumbar spine also showed no differences between the two groups.
At the end of the period of follow up the average CI score was 1.7+2.7 (range 0-12), with high comorbidity in 22.65% of our patients and no comorbidity, according to CI, in 73.2% of them. In terms of the appearance of other concomitant diseases, 18.3% of the individuals were diabetic, 32.4% had cardiovascular risk factors other than AHT, 14% developed ischemic cardiomyopathy, 12.7% cerebrovascular disease and the same percentage showed some degree of cognitive deterioration. On the other hand, 17% had at least one pathological osteoporotic fracture (8.5% vertebral, 5.3% femoral neck, 2.8% distal radial).
The genotype AA was found in 67.6%, SS in 2.8% and AS in 29.6% of the patients, with an allelic frequency A of 0.82 and S of 0.18, being in Hardy-Weinberg equilibrium (Χ2=0.03; p=0.8696 >0.05). The individuals with genotype AA, compared with the other genotypes (AS+SS), did not have more cardiovascular risk factors (29.2% vs 39%, p=0.4), cardiovascular disease (12.5% vs 17.4%, p=0.5), presence of comorbidity according to CI (27% vs 26%, p=0.9), higher mortality (18.8% vs 13%, p=0.5) or a greater number of pathological fractures overall (14.6% vs 21.7%, p=0.4) nor specific fractures (vertebral 6.3% vs 13%, p=0.3; femoral neck 6.3% vs 4.3%, p=0.6; distal radial 2.1% vs 4.3%, p=0.5).
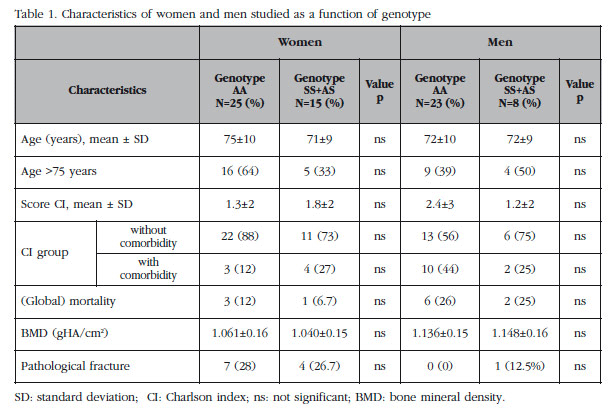
The average age of the women at the start of the study was 59+9.9 years, all postmenopausal, with a body mass index of 28±4. The average systolic arterial pressure was 155±22 mmHg and the diastolic was 93±10 mmHg. In the subgroup of women, those with the AA genotype had more pathological fractures, especially in the femoral neck, with no statistical association (12% vs 6.7%, p=0.5). The characteristics of the groups as a function of their sex and genotype is shown in Table 1.
Discussion
Calcium homeostasis plays a fundamental role in bone remodelling, and the alterations in the mechanisms involved in their regulation contribute to the development of pathology in bone metabolism [11]. The CaSR gene is a candidate gene for the determination of susceptibility to osteoporosis in AHT, the A986S locus of CaSR having been related to the maintenance of the concentration of extracellular ionic calcium within a narrow range [12], and with calciuria. The higher degree of elimination of calcium in patients with hypertension has been related with a decreased in BMD and, as a consequence, with a possible increase in the risk of fracture. On the other hand, AHT has been associated with raised levels of PTH, which accelerates bone remodelling, affecting both bone quality and mass [13].
Contrary to our expectations and consistent with a number of studies [7,14], we found no direct relationship between the genotypes of the A986S polymorphism and the incidence of pathological fractures, and no increase in the risk of pathological fractures in those patients who were carriers of allele S. Our data agree with a study previously published by our research group in which, in a population of women with hypertension, no differences were found in calcaemia, calciuria, levels of PTHi or BMD in the lumbar spine when categorised according to genotype AA or genotype AS+SS [15].
There are not many works which evaluate the influence of the A986S polymorphism on the risk of fractures. On the one hand, various studies carried out in European Caucasian populations found an association only in the young population[6], but not in postmenopausal women or in those with hypertension [11,15]. However, there are contradictory data in the literature. Thus, März et al.[16], did establish an association between the S allele and cardiovascular risk factors. Cetani et al. [11], investigating the effect of the polymorphism on fragility fractures in a group of 164 postmenopausal women, found no differences. Bollerslev et al. [17] analysed the association between polymorphism, bone mass and fractures in a cohort of 1,252 postmenopausal women without finding any relationship. This possible relationship has been analysed in other populations. Thus, Gianini et al. [18], studied 87 women subject to renal transplant and with persistent secondary hyperparathyroidism and found negative results similar to our group. Recently, an Italian group studied risk factors for vertebral fractures in a population with primary hyperparathyroidism. They studied 266 individuals (229 women and 37 men) with a genotype distribution similar to our group. They found that the presence of the S allele (evaluated as the AS+SS genotype) is associated with an increase in the risk of vertebral fractures with an odds ratio of 1.8 (95% CI: 1.1-2.9, p=0.05) after adjusting for age, sex, BMI, BMD and blood calcium. This population is different from ours, the key factor being the role of PTH regulated by the calcium sensor receptor [19].
The main limitation of our study is the sample size and the non-recording of morphometric fractures. Its strength comes from the homogeneity of the population studied, the long period of follow up and the use of an objective variable, as are clinical fractures.
In conclusion, the association between the genotypes of the A986S polymorphism of CaSR and the incidence of pathological fractures is difficult to establish and the different studies yield contradictory data, contributing to the increasing the confusion in this field. In our study we found no association between the A986S polymorphism of CaSR and clinical osteoporotic fractures, either in the general cohort or in the subgroup of women, although the etiopathogeny of osteoporosis in this population with hypertension can be determined by levels of blood calcium, hypercalciuria and the possibility of secondary hyperparathyroidism.
![]() Correspondence:
Correspondence:
Laisa Socorro Briongos-Figuero
Servicio de Medicina Interna
Hospital Universitario del Río Hortega
c/Dulzaina, 2
47012 Valladolid (Spain)
e-mail: laisadoc@hotmail.com
Date of receipt: 20/12/2014
Date of acceptance: 21/02/2015
Bibliography
1. Yang S, Nguyen ND, Center JR, Eisman JA, Nguyen TV. Association between hypertension and fragility fracture: a longitudinal study. Osteoporos Int 2014;25:97-103. [ Links ]
2. Perez-Castrillón JL, Martin-Escudero JC, Alvarez Manzanares P, Cortes Sancho R, Iglesias Zamora S, García Alonso M. Hypertension as a risk factor for hip fracture. Am J Hypertens 2005;18:146-7. [ Links ]
3. Gadallah M, Massry SG, Bigazzi R, Horst RL, Eggena P, Campese VM. Intestinal absorption of calcium and calcium metabolism in patients with essential hypertension and normal renal function. Am J Hypertens 1991;4:404-9. [ Links ]
4. Perez-Castrillon JL, Justo I, Silva J, Sanz A, Igea R, Escudero P, et al. Bone mass and bone modelling markers in hypertensive postmenopausal women. J Hum Hypertens 2003;17:107-10. [ Links ]
5. Tsuda K, Nishio I, Masuyama Y. Bone mineral density in women with essential hypertension. Am J Hypertens 2001;14:704-7. [ Links ]
6. Lorentzon M, Lorentzon R, Lerner UH, Nordstrom P. Calcium sensing receptor gene polymorphism, circulating calcium concentrations and bone mineral density in healthy adolescent girls. Eur J Endocrinol 2001;144:257-61. [ Links ]
7. Pratt JH, Ambrosius WT, Wagner MA, Maharry K. Molecular variations in the calcium-sensing receptor in relation to sodium balance and presence of hypertension in blacks and whites. Am J Hypertens 2000;13:654-8. [ Links ]
8. Brown EM, Gamba G, Riccardi D, Lombardi M, Butters R, Kifor O, et al. Cloning and characterization of an extracellular Ca(2+)-sensing receptor from bovine parathyroid. Nature 1993;366:575-80. [ Links ]
9. Yun FH, Wong BY, Chase M, Shuen AY, Canaff L, Thongthai K, et al. Genetic variation at the calcium-sensing receptor (CASR) locus: implications for clinical molecular diagnostics. Clin Biochem 2007;40:551-61. [ Links ]
10. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373-83. [ Links ]
11. Cetani F, Pardi E, Borsari S, Vignali E, Dipollina G, Braga V, et al. Calcium-sensing receptor gene polymorphism is not associated with bone mineral density in Italian postmenopausal women. Eur J Endocrinol 2003;148:603-7. [ Links ]
12. Cole DE, Peltekova VD, Rubin LA, Hawker GA, Vieth R, Liew CC, et al. A986S polymorphism of the calcium-sensing receptor and circulating calcium concentrations. Lancet 1999;353:112-5. [ Links ]
13. Cappuccio FP, Kalaitzidis R, Duneclift S, Eastwood JB. Unravelling the links between calcium excretion, salt intake, hypertension, kidney stones and bone metabolism. J Nephrol 2000;13:169-77. [ Links ]
14. Blázquez JA, Navarro L, Andrés C, Ontañón J, Navarro A, del Pino J. Relación entre el polimorfismo A986S del CaSR y presión arterial en mujeres postmenopáusicas recientes. Rev Esp Enf Metab Óseas 2008;17:137. [ Links ]
15. Pérez-Castrillón JL, Sanz A, Silva J, Justo I, Velasco E, Dueñas A. Calcium-sensing receptor gene A986S polymorphism and bone mass in hypertensive women. Arch Med Res 2006;37:607-11. [ Links ]
16. März W, Seelhorst U, Wellnitz B, Tiran B, Obermayer-Pietsch B, Renner W, et al. Alanine to serine polymorphism at position 986 of the calcium-sensing receptor associated with coronary heart disease, myocardial infarction, all-cause, and cardiovascular mortality. J Clin Endocrinol Metab 2007;92:2363-9. [ Links ]
17. Bollerslev J, Wilson SG, Dick IM, Devine A, Dhaliwal SS, Prince RL. Calcium-sensing receptor gene polymorphism A986S does not predict serum calcium level, bone mineral density, calcaneal ultrasound indices, or fracture rate in a large cohort of elderly women. Calcif Tissue Int 2004;74:12-7. [ Links ]
18. Giannini S, Sella S, Silva Netto F, Cattelan C, Dalle Carbonare L, Lazzarin R, et al. Persistent secondary hyperparathyroidism and vertebral fractures in kidney transplantation: role of calcium-sensing receptor polymorphisms and vitamin D deficiency. J Bone Miner Res 2010;25:841-8. [ Links ]
19. Eller-Vainicher C, Battista C, Guarnieri V, Muscarella S, Palmieri S, Salcuni AS, et al. Factors associated with vertebral fracture risk in patients with primary hyperparathyroidism. Eur J Endocrinol 2014;171:399-406. [ Links ]











 texto em
texto em