Meu SciELO
Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Revista de Osteoporosis y Metabolismo Mineral
versão On-line ISSN 2173-2345versão impressa ISSN 1889-836X
Rev Osteoporos Metab Miner vol.7 no.4 Madrid Nov./Dez. 2015
https://dx.doi.org/10.4321/S1889-836X2015000400006
General and bone pain syndrome in a patient treated with tenofovir
Síndrome general y dolores óseos en un paciente tratado con tenofovir
Arnaiz de las Revillas F.1, Marcote Ibáñez C.1, González-Lamuño D.2, García Unzueta M.3, Lino Montenegro E.4, Riancho J.A.1
1 Servicio Medicina Interna - Hospital Universitario Marqués de Valdecilla - Universidad de Cantabria - IDIVAL - Santander (España)
2 Servicio de Pediatría - Hospital Universitario Marqués de Valdecilla - Universidad de Cantabria - IDIVAL - Santander (España)
3 Servicio de Análisis Clínicos - Hospital Universitario Marqués de Valdecilla - Universidad de Cantabria - IDIVAL - Santander (España)
4 Gerencia de Atención Primaria - Servicio Cántabro de Salud - Santander (España)
SUMMARY
Tenofovir (TDF), is the only nucleotide analogue reverse transcriptase inhibitor for treating human immunodeficiency virus (HIV). Occasionally, it may cause acute renal failure and Fanconi syndrome.
We report the case of a 64-year-old male diagnosed with HIV infection 22 years previous and treated with tenofovir. In outpatient follow-up, the patient complained of progressive fatigue and diffuse aching bones. In several check-ups, increased alkaline phosphatase and parathyroid hormone (PTH) were observed. Over the past month, his condition worsened and he was admitted to hospital. Analytical data included marked glycosuria, hypophosphatemia, hyperphosphaturia and hypouricemia. All changes were resolved when TDF was discontinued.This illustrates the importance of clinical evaluations that include possible TDF-induced proximal tubulopathy in patients with general bone pain syndrome or mineral metabolism disturbances.
Key words: general syndrome, tenofovir, osteomalacia, Fanconi syndrome.
RESUMEN
El tenofovir (TDF), es el único inhibidor de la transcriptasa inversa análogo nucleótido para el tratamiento de la infección por virus de la inmunodeficiencia humana (VIH). Ocasionalmente, puede producir insuficiencia renal aguda y síndrome de Fanconi.
Presentamos el caso de un varón de 64 años con infección por VIH conocida desde hace 22 años, en tratamiento con tenofovir. En las revisiones ambulatorias refería un cuadro progresivo de astenia y dolores óseos difusos. En varias determinaciones se había observado una elevación de la fosfatasa alcalina y la paratohormona (PTH). Durante el último mes empeoró su estado, por lo que fue ingresado en el hospital. Entre los datos analíticos destacaban: glucosuria marcada, hipofosfatemia, hiperfosfaturia e hipouricemia. Todas las alteraciones se resolvieron tras suspender el TDF, lo que ilustra la importancia de que los clínicos incluyan la posibilidad de tubulopatía proximal por TDF en pacientes con dolores óseos, síndrome general o alteraciones del metabolismo mineral.
Palabras clave: síndrome general, tenofovir, osteomalacia, síndrome de Fanconi.
Introduction
Tenofovir (TDF), is the only inhibitor of nucleotide analogue reverse transcriptase for treatment ofhuman immunodeficiency virus (HIV) infection. It is sometimes associated with renal impairment, including tubular dysfunction and Fanconi syndrome [1,2]. This syndrome involves a defect in the transport of amino acids, glucose, phosphate, uric acid, potassium, bicarbonate and protein at proximal tubule level [3].
Case report
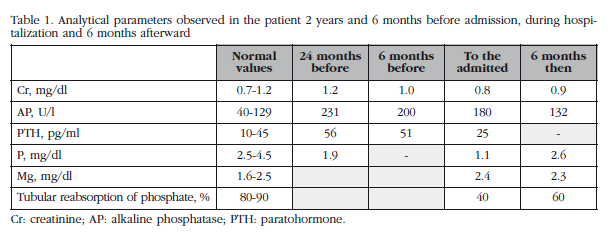
We report the case of a 64-year-old man diagnosed with HIV infection 22 years previous, taking didanosine, tenofovir and lopinavir/ritonavir. In outpatient revisions over the past two years, the patient complained of diffuse bone pain and fatigue. Several analyses detected elevated total and bone alkaline phosphatase (AP) and the parathyroid hormone (PTH). The 25-hydroxyvitamin D (25-HCC), total and ionized calcium and other routine biochemical parameters in serum and elementary and sediment in urine were normal (Table 1). Plain radiography of the spinal column showed degenerative signs and CT scan was normal.
During the last month, his condition worsened, with increased fatigue, loss of strength, difficulty walking and a loss of 7 kg so he was admitted to hospital. Physical examination was unremarkable except for predominantly proximal muscle weakness. The analytical data highlighted mild hyperglycemia (154 mg/dl), marked glycosuria (4+), hypophosphatemia, unusally high phosphaturia, hypouricemia (2 mg/dl), mild metabolic acidosis (bicarbonate 19 mmol/l) and (945 mg/24h). Furthermore, there was moderate aminoaciduria with increased glycine (x2), valine (x2), serine (x4) and threonine (x4) values. FGF23 serum levels were 6 pg /ml. Other parameters shown in Table 1.
From these results, incomplete Fanconi syndrome was diagnosed with probablesevere hypophosphatemia and osteomalacia linked to TDF. The drug was discontinued and treatment commenced with raltegravir and darunavir boosted with ritonavir. Furthermore phosphorus supplements, HCC-25 and 1.25-dihydroxyvitamin D (1.25 DHCC) were administered. His condition gradually improved with this regime. Six months after the withdrawal of TDF, bone pain and muscle weakness stopped, the patient had regained baseline weight and had normalized serum laboratory abnormalities, although phosphate reabsorption remained slightly low.
Discussion
The TDF is excreted by glomerular filtration and is actively transported by cells of the proximal tubule renal [1]. Although tubular disorders, including Fanconi syndrome, are a known complication of TDF treatment, acknowledged in its technical specifications and the subject of different publications [4,5], it is not often accompanied by clinical demonstrations [1,2]. Indeed, in a study of 422 patients with HIV infection, of which 381 received TDF, this drug was not found to be associated with globally altered levels of calcium, phosphorus, vitamin D or markers of bone remodeling, nor in bone mineral density (BMD) [6]. On the other hand, in a clinical trial involving 299 patients treated with TDF and followed up over 144 weeks, 10 cases of hypophosphatemia (similar to that found among stavudine frequency) were found, although none were necessary to remove the drug or developing Fanconi syndrome [7]. In that same study the authors found a slight decrease in spinal BMD, but not in the hip in those individuals treated with TDF. It has been suggested that concomitant administration of other drugs such as didanosine and lopinavir boosted with ritonavir, may increase the risk of tubulopathy [8,9].
Increased phosphate excretion with consequent hypophosphatemia was most relevant in this patient's evolution. The role of FGF23 in hypophosphatemia associated with PDT is controversial [10,11]. In our patient, FGF23 levels were decreased, which runs contrary to the implication of this phosphaturic factor and is consistent with a direct effect of the drug on the renal tubules. Although a bone biopsy was not carried out to confirm the accumulation of osteoid, clinical and laboratory manifestations, including increased PTH and FA are consistent with the existence of a hypophosphatemic osteomalacia [12,13]. The resolution after discontinuing TDF confirms the causal implication of this drug. However, this is a rare complication.
In a recent review of the literature, 53 cases of TFD-induced tubulopathy were found, of which 27 had bone changes consistent with osteomalacia. The median time from treatment initiation to the onset of renal impairment was 2.5 years [14]. The fact that these tubular alterations are not necessarily associated with a decreased glomerular filtration rate, next to that phosphatemia often not included in the biochemical parameters analyzed routinely, can lead to delays in diagnosis.
This case illustrates the importance of clinicians including the possibility of hypophosphatemia secondary to a proximal tubulopathy in the diagnosis of patients treated with vague TDF symptoms such as weakness or pain, which could otherwise be attributed to the underlying disease or other concomitant processes.
Competing interests
All authors declare no conflict of interest.
![]() Correspondence:
Correspondence:
Francisco Arnaiz de las Revillas
Hospital Universitario Marqués de Valdecilla
Servicio de Medicina Interna
Avda. Valdecilla, s/n
39008 Santander (España)
Correo electrónico:
farnaiz@humv.es
Date of receipt: 03/09/2015
Date of acceptance: 07/10/2015
Bibliogragraphy
1. Cooper RD, Wiebe N, Smith N, Keiser P, Naicker S. Systematic review and meta-analysis: renal safety of tenofovir disoproxil fumarate in HIV-infected patients. Clin Infect Dis 2010;51:496-505. [ Links ]
2. O'Donnell EP, Scarsi KK, Darin KM, Gerzenshtein L, Postelnick MJ. Low incidence of renal impairment observed in tenofovir-treated patients. J Antimicrob Chemother 2012;66:1120-6. [ Links ]
3. Asplin JR, Coe FL. Tubular disorders. In: Kasper D, Braunwald E, Fauci A, Hauser S, Longo D, Jameson J, editors. Harrison's Principles of Internal Medicine. 16th ed. New York: McGraw Hill; 2005. p. 1701. [ Links ]
4. Hall AM, Hendry BM, Nitsch D, Connolly JO. Tenofovir-associated kidney toxicityin HIV-infected patients: a review of the evidence. Am J Kidney Dis 2011;57:773-80. [ Links ]
5. Woodward CLN, Hall M, Williams IG, Madge S, Copas A, Nair D, et al Tenofovir-associated renal and bone toxicity. HIV Med 2009;10:482-7. [ Links ]
6. Samarawickrama A, Jose S, Sabin C, Walker-Bone K, Fisher M, Gilleece Y. No association between vitamin D deficiency and parathyroid hormone, bone density and bone turnover in a large cohort of HIV-infected men on tenofovir. J Int AIDS Soc 2014;17(4 Suppl 3):19568. [ Links ]
7. Gallant JE, Stazszewski S, Pzniak AL, De Jesus E, Suleiman JM, Miller MD, et al. Efficacy and safety of tenofovir DF vs stavudine in combination therapy in antiretroviral-naive patients:a 3-year randomized trial. JAMA 2004;292:191-201. [ Links ]
8. Gupta SK. Tenofovir-associated Fanconi syndrome: review of the FDA adverse event reporting system. Aids Patient Care STDS 2008;22:99-103. [ Links ]
9. Goicoechea M, Liu S, Best B, Sun S, Jain S. Greater Tenofovir-Associated Renal Function Decline with Protease Inhibitor-Based versus Nonnucleoside Reverse-Transcriptase Inhibitor-Based Therapy. J Infect Dis 2008;197:102-8. [ Links ]
10. Haverkort ME, Van der Spek BW, Lips P, Slieker WA, ter Heine R, Huitema AD, Bronsveld W. Tenofovir-induced Fanconi síndrome and osteomalacia in two HIV-infected patients: role of intracelular tenofovir diphosphate levels and review of the literatura. Scand J Infect Dis 2011;43:821-6. [ Links ]
11. Saeedi R, Jiang SY, Holmes DT, Kendler DL. Fibroblast grouth factor 23 is elevated in tenofovir-related hypophosphatemia. Calcif Tissue Int 2014;94:665-8. [ Links ]
12. Riancho JA. Osteomalacia y raquitismo. Rev Esp Enf Metab Oseas 2004;13:77-9. [ Links ]
13. Francis RM, Selby PL. Osteomalacia. Balliere's Clin Endocrinol Metab 1997;11:145-63. [ Links ]
14. Mateo L, Holgado S, Mariñoso ML, Perez-Andres R, Bonjoch A, Romeu J, Olive A. Hipophosphatemic osteomalacia induced by tenofovir in HIV infected patients. Clin Rheumatol 2014 May 3. (Epub ahead of print). [ Links ]











 texto em
texto em