Meu SciELO
Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Revista de Osteoporosis y Metabolismo Mineral
versão On-line ISSN 2173-2345versão impressa ISSN 1889-836X
Rev Osteoporos Metab Miner vol.8 no.2 Madrid Abr./Jun. 2016
Secondary hyperparathyroidism due to vitamin D deficiency
Hiperparatiroidismo secundario al déficit de vitamina D
López-Ramiro E.1, Rubert M.1, Mahillo I.2 and de la Piedra C.1
1 Laboratorio de Bioquímica Investigación
2 Departamento de Epidemiología.
IIS Fundación Jiménez Díaz - Madrid (España)
Work submitted as FEIOMM benefit the grant received to attend the 33rd Congress of the ASBMR (San Diego, 2011).
SUMMARY
Introduction: Vitamin D is increasingly recognized as playing a significant role in combatting many diseases. One is the development of secondary hyperthyroidism due vitamin D deficiency. To date, laboratory quantification methods of serum vitamin D were not well standardized. It could not be established with certainty from which levels of vitamin D certains abnormalities take place, like an elevation of PTH. The present study was aimed at determining below what vitamin D levels we will find abnormally high levels of PTH, carrying out the vitamin D determination in the laboratory with a standardized, reliable technique.
Methods: This descriptive, retrospective study was conducted with patients over 18 years in which determinations were made simultaneously with PTH, 25 (OH) vitamin D (25OHD) and which also have normal values of calcium, glomerular filtration rate and phosphorus.
For determining vitamin D, standardized electrochemiluminescence method was used with gas chromatography-mass spectrometry method. Using the Stava version 11 statistical program, the 25OHD was calculated where PTH value was above 70 pg/ml with greater sensitivity and specificity.
Results: In all, 4,083 patients were included, of whom 2,858 were women (70%) and 1,225 (30%) males. The mean age of the study population was 60.60 years (standard deviation, 15.29). 74% of the population had a serum PTH under 70 pg/ml (normal values) and 26% had a serum PTH higher tan 70 ng/ml. By constructing the ROC curve levels of 25OHD, depending on PTH values below or above 70 pg/ml, the area under the curve was 0.5962 (p<0.0001). The cut having jointly account the sensitivity and specificity that determined vitamin D levels to predict PTH values above 70 pg/ml was 24 ng/ml. Of the patients with normal PTH, 71% presented normal vitamin D values, while patients with elevated PTH (Greater than 70 pg/ml), almost half had a vitamin D below 24 ng / ml, which increased as the PTH percentage was elevated.
Conclusions: The 25OHD value that presents better specificity and sensitivity to predict abnormally high PTH is 24 ng/ml, which is higher than the level reported in previous work, (about 18 ng/ml) value. The results of this study, carried out with an appropriately calibrated method, showed that 44.9% of patients with vitamin D values of less than 24 ng/ml PTH had abnormally high levels, with a normal value of calcium and phosphorus and normal renal function. This percentage is less in those individuals between 18 and 40 years (24%) and reaches 49% beyond 60 years. These patients could be treated with vitamin D to prevent possible secondary hyperparathyroidism due to vitamin deficiency. It is noteworthy that the method of determining vitamin D used must be properly standardized with respect to gas chromatography-tandem mass spectrometry method.
Key words: secondary hyperparathyroidism, vitamin D deficiency, vitamin D standarization program, tandem-mass spectrometry, 25 (OH) vitamin D.
RESUMEN
Introducción: Cada vez está más demostrado el papel de la vitamina D en múltiples patologías, una de ellas el desarrollo de un hiperparatiroidismo secundario al déficit de vitamina D. Los métodos de laboratorio de cuantificación de vitamina D en suero no estaban bien estandarizados hasta ahora, por lo que no podía establecerse con certeza a partir de qué niveles de vitamina D se producían determinadas anomalías como la elevación de la PTH. Nuestro estudio pretende determinar por debajo de qué niveles de vitamina D nos encontraremos con niveles de PTH anormalmente elevados, realizando la determinación de vitamina D en el laboratorio con una técnica debidamente estandarizada y fiable.
Métodos: El estudio descriptivo y retrospectivo se realizó con pacientes mayores de 18 años en los que se hicieron simultáneamente determinaciones de PTH, 25(OH) vitamina D (25OHD) y que además tuvieran valores normales de calcio, filtrado glomerular y fósforo. Para la determinación de vitamina D se utilizó un método de electroquimioluminiscencia estandarizado con respecto a la técnica de gases-masas. Por el programa estadístico Stava versión 11 se calculó el valor de 25OHD para el que la PTH se elevaba por encima de 70 pg/ml con la mayor sensibilidad y especificidad.
Resultados: Se incluyeron 4.083 pacientes, de los que 2.858 eran mujeres (70%) y 1.225 (30%) varones. La edad media de la población estudiada fue 60,60 años (desviación estándar, 15,29). El 74% de la población tenía una PTH en suero por debajo de 70 pg/ml (valores considerados normales) y el 26% mayor de 70 pg/ml. Al construir la curva de ROC de los niveles de 25OHD, en función de valores de PTH por debajo o por encima de 70 pg/ml, el área bajo la curva fue 0,5962 (p<0,0001). El punto de corte teniendo en cuenta conjuntamente la sensibilidad y la especificidad que determinaban los valores de vitamina D para predecir los valores de PTH por encima de 70 pg/ml fue 24 ng/ml. De los pacientes con PTH normal, el 71% tenían valores de vitamina D normales, mientras que, de los pacientes con PTH elevada (mayor de 70 pg/ml), casi la mitad presentaban una vitamina D menor de 24 ng/ml, porcentaje que aumentaba según se iba elevando la PTH.
Conclusiones: El valor de 25OHD que muestra una mejor especificidad y sensibilidad para predecir valores anormalmente elevados de PTH es 24 ng/ml, valor superior al presentado en trabajos anteriores (alrededor de 18 ng/ml).
Con los resultados de este estudio, realizado con un método debidamente calibrado, se puede decir que el 44,9% de pacientes con valores de vitamina D menores de 24 ng/ml presenta niveles de PTH anormalmente elevados, con una función renal normal y valores de calcio y fósforo normales. Este porcentaje es menor entre los 18 y 40 años (24%) y llega al 49% por encima de los 60 años. Estos pacientes podrían tratarse con vitamina D para evitar un posible hiperparatiroidismo secundario al déficit de dicha vitamina. Es importante tener en cuenta que el método de determinación de vitamina D utilizado debe estar debidamente estandarizado con respecto al método de gases-masas.
Palabras clave: hiperparatiroidismo secundario, déficit de vitamina D, vitamin D standarization program, espectrometría tándem-masas, 25(OH) vitamina D.
Introduction
Vitamin D is involved in the metabolism of phosphorus and calcium. Vitamin D deficiency is associated with osteoporosis and osteomalacia in adults and rickets in children. Recent studies have also shown their role in autoimmune diseases, cancer, cardiovascular disease, etc. [1-4].
PTH is a hormone secreted by the parathyroid gland, involved in calcium metabolism, bone obtaining it if hypocalcemia, and increasing the production of 1,25(OH)2 vitamin D in the kidney, to promote absorption of calcium. Moreover, it also increases renal tubular reabsorption of calcium.
In the clinical laboratory, we often find PTH higher than the upper normal limit, but with normal creatinine or glomerular filtration, calcium and phosphorus and which therefore do not correspond to a primary or secondary hyperparathyroidism, data mystifying the clinician. In many of these cases, the increase in PTH is associated with vitamin D deficiency, made increasingly found in our population and that has not been paid much attention [5-10]. Vitamin D levels are measured through levels of metabolite 25(OH) vitamin D (25OHD), which express the status of this vitamin in patients [11]. Until recently, most 25OHD determinations were not adequately standardized and values varied greatly depending on the different methods [12].
Moreover, it is of great interest to know at what 25OHD levels an abnormal elevation of PTH occurs. This may help us rule out other likely causes of hyperparathyroidism [13].
Several studies show considerable variation between 25OHD results obtained in the laboratory by different methods: radioimmunoassay, electrochemiluminiscence, HPLC and tandem-mass spectrometry [9,10,14-16]. The variability between laboratories methods leads to a misallocation of patients [17], as well as a lack of standards when applying a health policy [18]. All this makes it difficult to establish normal vitamin D levels and beyond which it is likely that an abnormal increase in PTH occurs.
Because of this a program of international standardization (Vitamin D Standardization Program) has been launched in collaboration between the NIH-ODS (National Institutes of Health, Office of Dietary Supplements), Centers for Disease Control and Prevention (DCP) and the National Institute of Standards and Technology (NIST). This has led to the emergence of reference calibrators available to manufacturers so that the values obtained are the same for all methods [12,18]. Reference gauges are validated against liquid chromatography technique/tandem-mass, which is certainly the most accurate of those currently available.
The aim of this study is to determine the 25OHD value below which PTH abnormally increased. It is important to use a method of determining 25OHD properly standardized with respect to the gas-mass method, which is the gold standard, since most of the papers published so far in the literature are carried out with methods of determining 25OHD that are not properly standardized.
Materials and methods
A retrospective, cross-sectional, descriptive study was carried out at the Jimenez Diaz Foundation Hospital Biochemistry Laboratory (Madrid).
Consecutive blood tests were analyzed of the patients who had been asked for PTH, 25OHD, Ca and glomerular filtration rate (GFR) simultaneously during the period from May 2012 to November 2012 in the hospital.
Only those patients over 18 with FG greater than 60 and serum calcium between 8.4 mg/dl and 10.5 mg/dl simultaneously were included. Therefore, all patients with renal failure and abnormal calcium levels were excluded to rule out primary hyperparathyroidism or secondary to kidney failure.
A database was created containing all coded patients in which different variables were included in the study (age, sex, and analytical parameters) obtained manually through data from the clinical laboratory Hospital Foundation Jimenez Diaz.
This study was approved by the Ethics Committee of Health Research Institute of the FJD. As the data were drawn from a general Biochemistry Laboratory database, without using patients' names, informed consent was not required.
PTH determination was carried out in an automatic apparatus electrochemiluminescence ADVIA Centaur (SIEMENS). PTH levels greater than 70 pg/ml were considered abnormal. The reference range provided by the company was 14-70 pg/ml. As with the remaining second generation methods, it measures intact hormone 1-84, with the crosstalk of the truncated PTH at amino-terminal level. The sensitivity of the method is 5 pmol/ml and the coefficients of intra- and inter-assay variation are <7% and <10% respectively.
The 25OHD determination was carried out by electrochemiluminescence in an iSys autoanalyzer (IDS, UK), a method of determining properly standardized vitamin with respect to the gas-mass method. The sensitivity of the method is 4 ng/ml and the coefficients of variation intra- and inter-assay are <5% and <7%, respectively.
Using the statistical software Stava version 11, the 25OHD value was calculated for which PTH rose more than 70 pg/ml with a sensitivity and greater specificity, calculating the area under the ROC curve and checking that it was statistically significant.
A descriptive analysis of the sample by calculating the percentage of men and women, the mean and median age was also performed. The percentages were also calculated of patients with PTH above and below 70 pg/ml and the percentage of patients with 25OHD with different values (patients with vitamin D under 10, 10 to less than 24, 24 to less than 30 and patients with values greater than 30).
The differences between individuals with PTH higher and lower than 70 pg/ml were described using the Student t test, Mann-Whitney and Chi-square.
Results
A total of 9,225 patients were studied, of whom 5,142 were excluded for being under age, presenting kidney failure or abnormal calcium levels. Therefore, only a total of 4,083 patients, 2,858 women (70%) and 1,225 (30%) men, all over 18 years old were included in the study.
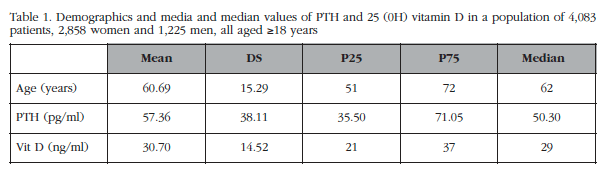
The average age of the population was 60.60 years with a standard deviation of 15.29, and the median age was 62 years. The minimum age was 18 years and the maximum age of 100 years. 74% of the population had a PTH below or equal to 70 pg/ml (normal values) and 26% greater than 70 pg/ml. The demographics of the patients are shown in table 1.
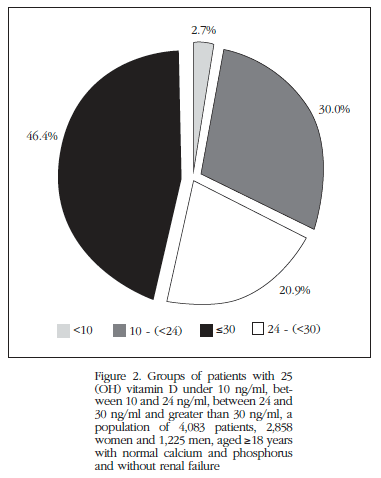
Regarding 25OHD levels of our population, only 46.4% of the same showed that levels above 30 ng/ml metabolite, 20.9% had levels between 24 and 30 ng/ml, 30% from 10 to 23 ng/ml, and 2.7% levels <10 ng/ml (Figure 2).
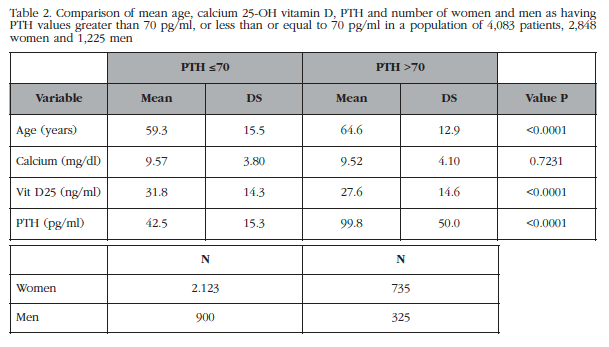
The baseline description of the sample showed no clinically significant differences in terms of PTH values depending on sex (Table 2). However, if there is significant age difference between patients with normal and abnormal values of PTH (59.3±15.5 years vs 64.6±13.9 years, p<0.001), with older patients with abnormal PTH and also presenting 25OHD values significantly lower than in the group with normal PTH (Table 2).
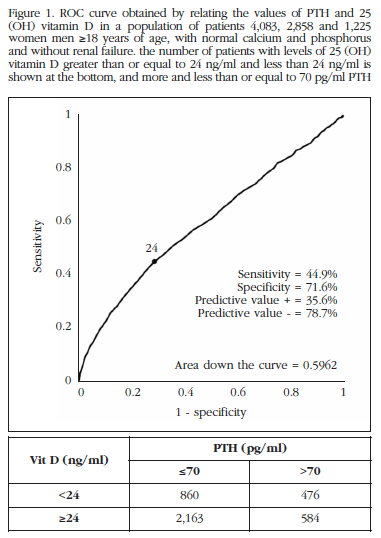
With patient data we construct the ROC curve for 25OHD levels in terms of having PTH values below or above 70 pg/ml. An area below the curve of 0.5962 (p<0.0001) was obtained, which shows that there is a relationship between 25OHD and PTH (Figure 1).
When using vitamin D values for predicting PTH values above 70 pg/ml, the best cutoff point was 24 ng/ml (Figure 1) considering sensitivity and specificity jointly.
In our population, 32.7% of the sample were found to have vitamin D levels less than 24 ng/ml, which were 44.9% of PTH values greater than 70 pmol/ml. By dividing the patients into 3 age groups: 18 to 40 years between 40 and 60 years and older than 60, a remarkable fact was observed. In the 18 to 24 year group, of the patients with 25OHD less than 24 ng/ml, only 24% had values of PTH >70 pg/ml. Among patients 40-60 years, 33.7% of patients with low levels of 25OHD had elevated PTH value. Finally, in the group of older patients (beyond 60 years), 49% of the patient group with PTH >70 pg/ml and 25OHD <25 ng/ml was shown. Thus, as people reach an advanced age, the likelihood that a low 25OHD level produces a high figure of PTH is higher. There is not the same risk at all ages. It is also interesting to note that, in our work, the percentage of patients with 25OHD <24 ng/ml is 32.7%, and the percentages are similar in the different age ranges: 33.2%, 31.7% and 32%, respectively. That is, in our population we found that there is a higher percentage of patients with low 25OHD levels among those over 60 years.
Discussion
Our work shows an inverse association between serum 25OHD and PTH. Other authors have observed this correlation [19,21].
The value that maximizes 25OHD specificity and sensitivity, as to produce an abnormal increase in PTH is 24 ng/ml, greater than presented in previous work value, which was about 18 ng/ml [22].
Since this study corroborates the fact that a vitamin D deficiency may cause an abnormal elevation of PTH values, we consider it important, in clinical practice, to determine levels of 25OHD in those patients who present abnormally high PTH values without known cause, to rule out hyperparathyroidism secondary to vitamin D deficiency. This could be corrected by supplementing patients with the necessary vitamin D, thus avoiding the aggravation of diseases caused by elevated PTH, as is the case of osteoporosis [19].
The problem with studies until now is that they have used different methods of determining 25OHD which were not properly standardized, so there was a great variation between the results of 25(OH)D obtained. The levels of vitamin D considered normal were difficult to establish as were those from which it was likely that an abnormal increase in PTH occurred. The different laboratory techniques used to determine vitamin D are: radio-immunoassay, electrochemoluminiscence, HPLC or lipid chromatography tandem-mass spectrometry. Currently the most accurate technique is the liquid/tandem-mass chromatography, which undoubtedly is the most accurate of those available [11] and no validated reference calibrators against this technique. In this study we used an electrochemiluminescence in an IDS-iSYS (UK) autoanalyzer, properly standardized gas-mass relation, so the results are considered valid, and comparable to studies with other methods that are well calibrated.
In most studies on vitamin D to date, the method used is not discussed, nor do they specify whether the method is calibrated with respect to the gas-mass technique. Thus it is impossible to know whether the results of the values vitamin D found in the studies are correct and whether they could be extrapolated to the general population and applied to clinical practice once the clinician finds certain values in analysis of patients.
In a group of postmenopausal women, Capatina et al. [23] observed that 27.2% of patients with vitamin D deficiency had secondary hyperparathyroidism, a lower percentage than what we found, 44.9%. Laroche et al. [24] observed that 13% of patients with 25OHD <30 ng/mL had secondary hyperparathyroidism. However, Sadat Ali et al. [25] found in 200 patients (150 women and 50 men aged 18 to 69 years) all of whom presented vitamin D deficiency, determined by gas-mass, had secondary hyperparathyroidism.
As mentioned above, the results of this study, a properly calibrated method, show that 44.9% of patients with 25OHD levels <24 ng/mL had serum PTH values >70 pg/ml, no other proper reasons. This percentage is lower in the population between 18 and 40 years and more in the population >60 years. It seems, therefore, important that the clinician concerned with vitamin D to patients with 25OHD levels <24 ng/ml in order to avoid possible secondary hyperparathyroidism deficit of vitamin D. In order to establish a cut-off, it is important that 25OHD determination methods are properly standardized with respect to the gas-mass technique.
Competing interests: The authors declare no conflicts of interest.
![]() Correspondence:
Correspondence:
Concha de la Piedra
Fundación Jiménez Díaz
Avda. Reyes Católicos, 2
28040 Madrid (España)
e-mail: cpiedra@fjd.es
Date of receipt: 22/11/2015
Date of acceptance: 16/03/2016
Bibliography
1. Quesada Gómez JM, Navarro Valverde C. Niveles inadecuados de vitamina D: no es una D-liciosa perspectiva. Rev Osteoporos Metab Miner. 2013;5:65-6. [ Links ]
2. Shoji T, Nishizawa Y. Chronic kidney disease (CKD) and bone. Pleiotropic actions of vitamin D and survival advantage. Clin Calcium. 2009;19:514-21. [ Links ]
3. Rubert M, Montero M, De la Piedra C. Niveles muy descendidos de 25(OH) vit D en pacientes sometidos a cirugía bariátrica. Rev Esp Enfer Metab Óseas. 2007;16:103. [ Links ]
4. Rojas Rivera J, De la Piedra C, Ramos A, Ortiz A, Egido J. The spanding spectrum of biological actions of vitamin D. Nephrol Dial Transplant. 2010;25:2850-65. [ Links ]
5. Muñoz-Torres M, Sosa Henríquez M. Situación actual de los niveles de vitamina D en la población española. Rev Esp Enfer Metab Óseas. 2005;14 (Supl.1):17-20. [ Links ]
6. Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266-81. [ Links ]
7. Van Schoor NM, Lips P. Worldwide vitamin D status: Best Pract Res Clin Endocrinol Metab. 2011;25:671-80. [ Links ]
8. Lips P, Duong T, Oleksik A, Black D, Cummings S, Cox D, et al. A global study of vitamin D status and parthyroid function in postmenopausal women with osteoporosis: baseline data from the multiple outcomes for raloxifene evaluation clinical trial. J Clin Endocrinol Metab. 2001;86:1212-21. [ Links ]
9. Valcour A, Blocki F, Hawkins DM, Rao SD. Effect of age and serum 25-OH vitamin D on serum parathyroid hormone levels. J Clin Endocrinol Metab. 2012;97:3989-95. [ Links ]
10. Garg MK, Tandom N, Marwaha RK, et al. The relationship between serum 25-hydroxy vitamin D.parathormone and bone mineral density in Indian population. Clin Endocrinol (Oxf). 2014;80:41-6. [ Links ]
11. Ofenloch-Haehnle B. Approaches to measurement of Vitamin D concentrations-immunoassays. Scand J Clin Lab Invest Suppl. 2012;243:50-3. [ Links ]
12. Thienpont LM, Stepman CM, Vesper HW. Standarization measurements of 25-hydroxyvitamin D3 and D2. Scand J Clin Lab Invest Suppl. 2012:243:41-9. [ Links ]
13. Ortigosa Gómez S, García-Algar O, Mur Sierra A, Ferrer Costa R, Carrascosa Lezcano A, Yeste Fernández D. Concentraciones plasmáticas de 25-OH vitamina D y parathormona en sangre de cordón umbilical. Rev Esp Salud Pública. 2015;89:75-83. [ Links ]
14. Kushnir MM, Ray JA, Rockwood AL, Roberts WL, La'ulu SL, Whittington JE, et al. Rapid analysis of 25-Hydroxyvitamin D2 and D3 by Liquid Chromatography-Tandem Mass Spectrometry and Association of Vitamin D and Parathyroid Hormone Concentrations in Healthy Adults. Am J Clin Pathol. 2010;134:148-56. [ Links ]
15. Moon HW, Cho JH, Hur M, Song J, Oh GY, Park CM, et al. Comparison of four current 25-hydroxyvitamin D assays. Clin Biochen. 2012;45:326-30. [ Links ]
16. Binkley N, Krueger DC, Morgan S, Wiebe D. Current status of clinical 25-Hydroxivitamin D measurement: an assessment of between-laboratory agreement. Clin Chim Acta. 2010;411:1976-82. [ Links ]
17. Binkley N, Krueger D, Cowgill CS, Plum L, Lake E, Hansen KE, et al. Assay variation confounds the diagnosis of hypovitaminosis D: a call for standardization. J Clin Endocrinol Metab. 2004;89:3152-7. [ Links ]
18. Barake M, Daher RT, Salti I, Cortas NK, Al-Shaar L, Habib RH, et al. 25-Hydroxivitamin Dassay variations and impact on clinical decision making. J Clin Endocrinol Metab. 2012;97:835-43. [ Links ]
19. Zhang Q, Shi L, Peng N, Xu S, Zhang M, Zhang S, et al. Serum concentrations of 25- hydroxyvitamin D and its association with bone mineral density and serum parathyroid hormone levels during winter in urban males for Guiyan, Southwest China. Br J Nutr. 2016;4:1-7. [ Links ]
20. Olmos JM, Hernández JL, García Velasco P, Martínez J, Llorca J, González-Macías J. Serum 25-hydroxyvitamin D, parathyroid hormone, calcium intake and bone mineral density in Spanish adults. Osteoporosis Int. 2016;27:105-13. [ Links ]
21. El Badawy AA, Aboserea MM, El Seifi OS, Mortada EM, Bakry HM, Waly EH, et al. Vitamin D, parathormone and associated minerals among students in Zagazig district, Sharkia Governorate, Egypt. Int J Vitam Nutr Res. 2014;84:3-4. [ Links ]
22. Hawkins F. La vitamina D y el hueso. Rev Esp Enf Metab Óseas. 2007;16:45-7. [ Links ]
23. Capatina C, Carsote M, Caragheorgheopol A, Poiana C, Berteanu M. Vitamin D deficiency in postmenopausal women- biological correlates. Maedica (Buchar). 2014;9:316-22. [ Links ]
24. Laroche M, Nigon D, Gennero I, Lassoued S, Pouilles JM, Trémolières F, et al. Vitamin D deficiency prediction by patient questionnairy and secondary huperparathyroidism in a cohort of 526 healthy subjects in their fifties. Presse Med. 2015;44(7-8):e283-90. [ Links ]
25. Sadat-Ali M, Al-Omran AS, Al-Turki HA. Parathyroid glands respose to low vitamin D levels in healthy adults: a cross-sectional study. Ulster Med J. 2015;84:26-9. [ Links ]











 texto em
texto em