Meu SciELO
Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Revista de Osteoporosis y Metabolismo Mineral
versão On-line ISSN 2173-2345versão impressa ISSN 1889-836X
Rev Osteoporos Metab Miner vol.15 no.4 Madrid Out./Dez. 2023 Epub 19-Fev-2024
https://dx.doi.org/10.20960/revosteoporosmetabminer.00025
CASE REPORT
Calcinosis cutis
1Servicio de Medicina Interna. Hospital Universitario Marqués de Valdecilla-Instituto de Investigación Sanitaria Valdecilla (IDIVAL). Departamento de Medicina y Psiquiatría. Universidad de Cantabria. Santander
2Servicio de Radiodiagnóstico. Hospital Universitario Marqués de Valdecilla. Universidad de Cantabria. Santander
Case report:
we report the case of a 48-year-old woman with pain in the maxillary sinuses and temporal areas. The presence of subcutaneous facial calcific plaques was confirmed in computed tomography (CT). Both the physical examination and the lab test results were within normal limits. Upon further questioning, the patient mentioned that she had been administered a facial filler product containing calcium hydroxyapatite (CaHA) (Radiesse®) the year before.
Discussion:
CaHA microspheres are radiopaque, making them visible through conventional x-rays, especially CT scans. The characteristic imaging features, typically bilateral and separate from the bone, along with the history of previous injection of this material, should help the clinician recognize this finding and isolate it from other conditions and diseases. Because of the popularity of this facial rejuvenation technique, clinicians should be familiar with the imaging characteristics associated with the deposition of this substance.
Keywords: Calcinosis; Calcium hydroxyapatite; Radiesse®
INTRODUCTION
Calcinosis cutis is a rare disorder characterized by insoluble calcium salt deposition in cutaneous and subcutaneous tissues. This disorder can be due to multiple processes, including connective tissue diseases, tumors, and trauma. Patients may present with visible skin abnormalities such as papules, nodules, or plaques, which can sometimes ulcerate, releasing some sort of whitish material, or may be incidentally diagnosed through imaging modalities or histological findings (1). We hereby report the case of a woman with subcutaneous facial calcifications associated with the administration of a biodegradable and resorbable filler containing calcium hydroxyapatite microspheres (Radiesse®).
CASE REPORT
We report the case of a 48-year-old woman referred to our hospital with oppressive pain in both maxillary sinuses and temporal areas over the past few months, radiating across her entire face. She was initially evaluated by the Otorhinolaryngology unit that ordered a facial and paranasal sinus computed tomography (CT). The CT scan revealed the presence of calcific plaques of facial subcutaneous fat location spreading from the nasal commissures towards the auricular region on both sides (Figs. 1 A and B), which triggered her referral to our center.
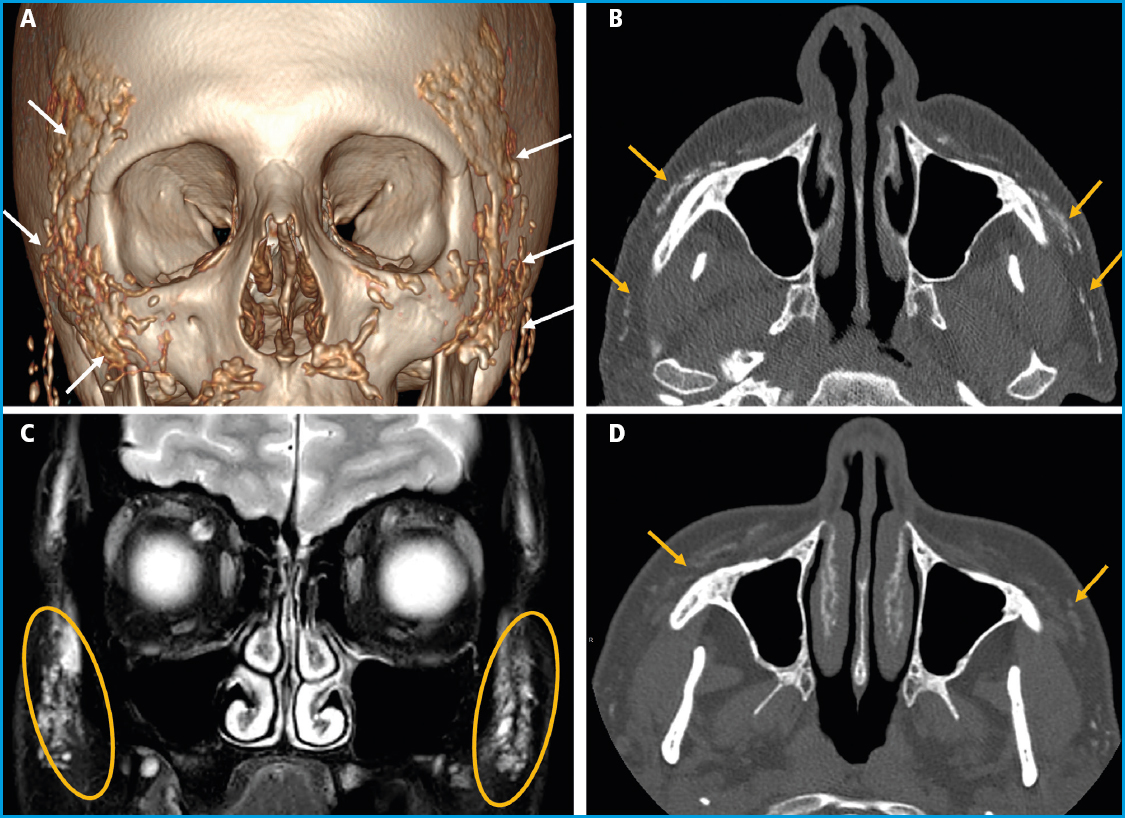
Figure 1 A. 3D reconstruction of a facial CT scan. B. Axial image of a facial CT scan without contrast. C. Coronal image of a T2-weighted fat-saturated magnetic resonance imaging (MRI) sequence. D. Axial image of a facial CT scan without contrast 3 years after the Radiesse® injection. Multiple hyperdense foci are evident in the CT scans (A and B), diffusely distributed across the subcutaneous cellular tissue of, predominantly, the zygomatic, infraorbital, and buccal regions, corresponding to calcium hydroxyapatite deposits. The MRI (C) shows these deposits overtly hyperintense on the T2-weighted imaging. Significant product resorption can be seen after 3 years (D), with thin residual deposits.
The patient's past medical history included smoking in her youth, but no other known toxic habits or allergies. She had a history of hypercholesterolemia, migraines, and adjustment disorder, left intercostal neuropathy, and left eye amblyopia. Six years prior, she had been diagnosed with a tubular carcinoma in her left breast, which was successfully treated with surgery (tumorectomy), radiation therapy, and hormone therapy. She was currently on drugs, including desloratadine, almotriptan, diazepam, desvenlafaxine, mirtazapine, paracetamol, and amitriptyline. The physical examination was normal. Complete blood count, erythrocyte sedimentation rate (ESR), routine biochemistry including calcium, phosphate, magnesium levels, proteinogram, and urine element and sediment analysis all fell within normal limits. Ionized calcium, 25-hydroxy-vitamin D (25(OH)D), parathyroid hormone (PTH), procollagen type 1 N-terminal propeptide (P1NP), C-terminal telopeptide of type 1 collagen (CTX), and angiotensin-converting enzyme (ACE) levels also fell within normal ranges. Upon further inquiry, the patient mentioned that she had been treated with a dermal filler product (Radiesse®) for wrinkle correction in the malar, labial commissure, and chin areas one year prior. Also, that this procedure was repeated 3 months later, being the product also administered in both temporal regions. Three years later, a new follow-up imaging study confirmed the clear reduction of calcium deposits in the facial area (Figs. 1 C and D).
DISCUSSION
Radiesse® (Merz Pharma GmbH & Co. KGaA, Frankfurt, Germany) is a biodegradable resorbable filler that contains calcium hydroxyapatite (CaHA) microspheres suspended in a carrier gel to stimulate the endogenous production of collagen (2). Experimental research on animals has demonstrated that this neocollagenesis appears on week 4 and goes on for, at least, 12 months after the injection (3). In fact, in individuals receiving these fillers, the effects of CaHA injections remain visible for nearly 18 months.
Since it was approved by the Food and Drug Administration (FDA) back in 2006, CaHA has been used in plastic and reconstructive surgery to augment the deep dermal and subdermal soft tissue of the facial area, smoothing out wrinkles. It is also used to restore or correct the signs of facial fat loss (lipoatrophy) in individuals infected with the human immunodeficiency virus (HIV), and for soft tissue augmentation in other cutaneous areas (neck, arms, buttocks, etc.) (4). Good results have been documented in the medical literature available after the use of CaHA, and patient satisfaction scores are high. Also, CaHA has a good safety profile, although transient adverse events such as bruising, swelling, redness, pain, and itching at the injection site have been reported. Also, in up to 3% of the cases, nodules can become evident, which in most cases are not visible and resolve without further treatment (5).
The calcium present in CaHA microspheres makes them radiopaque. However, a trial conducted back in 2008 (6) demonstrated that CaHA is not always visible in conventional x-rays, while indeed it is easily recognizable in CT scans immediately after the injection. A similar phenomenon occurs when analyzing magnetic resonance imaging (MRI), where CaHA deposits appear as a low-to-moderate intensity signals (7) that often disappears 2 and a half years later.
Therefore, we should understand the characteristics of CaHA deposit images to differentiate them from other conditions that have a similar radiographic appearance, such as myositis ossificans, dystrophic calcifications, milia-like osteomas of the skin, and foreign bodies (8). However, CaHA deposits should not pose any diagnostic challenges, especially if the radiologist knows the patient's health record. The traditionally bilateral presence of the material, which separates from the bone, along with a history of prior injection of this product, should help the clinician make accurate diagnoses. There is no evidence that CaHA migrates or that osteogenesis is stimulated after placing the filler at deep dermis and subcutaneous level. However, we should mention that, over time, the resorption of CaHA microspheres can lead to a reduced radiodensity of the filler material (6).
The popularity of soft tissue fillers for facial rejuvenation has increased significantly over the past few years. In fact, dermal fillers have become one of the most popular clinical esthetic therapies, with 2.6 million injections administered in the United States alone back in 2018 (9). The popularity of soft tissue fillers is partly due to being a quick, less invasive, and technically less complex procedure compared to surgery. In 2018, the most widely used fillers in the United States were hyaluronic acid and CaHA (9). This type of esthetic treatment has also gained popularity in our country (10). Therefore, clinicians should become familiar with the imaging characteristics associated with the deposition of this substance. The case presented here should help clinicians recognize this finding and differentiate it from other conditions and diseases.
BIBLIOGRAFÍA/REFERENCES
1. E TY, Yang X-J, Bi C, Xue F, Cao Y-Q. Idiopathic calcinosis cutis of the buttocks:A case report and review of the literature. Medicine 2023;102:15(31129). DOI:10.1097/MD.0000000000031129 [ Links ]
2. Sadick NS, Katz BE, Roy D. A multicenter, 47-month study of safety and efficacy of calcium hydroxylapatite for soft tissue augmentation of nasolabial folds and other areas of the face. Dermatologic Surg 2007;33(Suppl. 2):S122-7. DOI:10.1111/j.1524-4725.2007.33351.x [ Links ]
3. Coleman KM, Voigts R, DeVore DP, Termin P, Coleman WP. Neocollagenesis after injection of calcium hydroxylapatite composition in a canine model. Dermatologic Surg 2008;34(Suppl 1):53-5. DOI:10.1111/j.1524-4725.2008.34243.x [ Links ]
4. De Almeida AT, Figueredo V, Da Cunha ALG, Casabona G, Costa De Faria JR, Alves EV, et al. Consensus Recommendations for the Use of Hyperdiluted Calcium Hydroxyapatite (Radiesse) as a Face and Body Biostimulatory Agent. Plast Reconstr Surg - Glob Open 2019;7(3):1-9. DOI:10.1097/GOX.0000000000002160 [ Links ]
5. Kadouch JA. Calcium hydroxylapatite:A review on safety and complications. J Cosmet Dermatol 2017;16(2):152-61. DOI:10.1111/jocd.12326 [ Links ]
6. Carruthers A, Liebeskind M, Carruthers J, Forster BB. Radiographic and computed tomographic studies of calcium hydroxylapatite for treatment of HIV-associated facial lipoatrophy and correction of nasolabial folds. Dermatologic Surg 2008;34(Suppl 1):78-84. DOI:10.1111/j.1524-4725.2008.34247.x [ Links ]
7. Pavicic T. Complete biodegradable nature of calcium hydroxylapatite after injection for malar enhancement:An mri study. Clin Cosmet Investig Dermatol 2015;8:19-25. DOI:10.2147/CCID.S72878 [ Links ]
8. Valiyaparambil J, Rengasamy K, Mallya SM. An unusual soft tissue radiopacity - Radiographic appearance of a dermal filler. Br Dent J 2009;207(5):211-2. DOI:10.1038/sj.bdj.2009.764 [ Links ]
9. Corduff N, Chen JF, Chen YH, Choi HS, Lam Y, Lesthari NI, et al. Pan-Asian Consensus on Calcium Hydroxyapatite for Skin Biostimulation, Contouring, and Combination Treatments. J Clin Aesthet Dermatol 2021;14(8):E76-85. [ Links ]
10. Amselem M. Radiesse®:A novel rejuvenation treatment for the upper arms. Clin Cosmet Investig Dermatol 2015;9:9-14. DOI:10.2147/CCID.S93137 [ Links ]
Received: May 04, 2023; Accepted: July 10, 2023











 texto em
texto em 


