INTRODUCTION
The importance of the problem of finding effective treatment and prophylaxis for influenza and other acute respiratory viral infections is due to their mass, high susceptibility of the population to this, and a significant percentage of complications. Every year in Ukraine, from 10 to 14 million people suffer from them, which accounts for 25-30% of the total incidence. Mortality from these diseases and their complications has no tendency decrease, on the contrary, stabilization is noticeable, and even increase [1]. Although the mortality rate from acute respiratory viral infections (ARVI) in the United States is 0.01%, there is a tendency to increase this indicator [2, 3, 4]. Despite the significant number of developed antibacterial drugs, unsatisfactory treatment results are noted. In connection with the rapidly progressing inflammatory process in the lung parenchyma, which is accompanied by severe intoxication, respiratory and cardiovascular failure, in 0.1-2% of cases, disruption of the adaptive-adaptive occurs leading to the death [5]. Deterioration of the Ecological background, decrease in reactivity and significant allergization of the population do not always allow the use of an optimal antibiotic. This situation, combined with the significant resistance of microflora to certain drugs, becomes a prerequisite for the ineffective treatment of acute and development of protracted pneumonia in 22.7-38.4% of patients. The spectrum of etiological factors that cause acute damage to the respiratory canal and lung parenchyma is very significant. The etiology of acute respiratory viral infections is often caused by influenza A and B viruses, respiratory syncytial virus, herpes virus, and rhinovirus. The issues of searching drugs for the prevention and treatment of influenza and acute respiratory viral infections remain relevant, especially at an early stage, given the limited capabilities of medical practitioners in the differential diagnosis of these diseases at the prehospital stage. In the last decade, a significant therapeutic anti-influenza effect of proteolysis inhibitors has been established .Previous report revealed the inhibition of the reproduction of the virus in the tissue and lung culture of mice [6]. In addition, a reduction in the mortality rate in the case of the lethal from of influenza and acute respiratory diseases (ARI) in children has been also observed [7]. In our previous studies, it was experimentally established that a cell inhibitor of trypsin-like proteinases blocked proteinase, preventing influenza diseases expression. Our studies also observed that animals remained alive even after a lethal dose of the influenza virus was used [8]. It is important that at the same time other systems, primarily immunity, did not participate in the process of anti-infection protection. From, we obtained an antiferment-inhibitor of trypsin-like proteinases with a high antiviral property from waste human blood donation [9]. In addition, we received an antiviral drug, a trypsin-like proteinase inhibitor, from human donated blood waste, which protects human from flu [10]. The search for material from which it is possible to obtain a drug presumably with antiviral activity is carried out by other research institutes, the materials of which were provided to us for testing.
Objective: Nowadays, the research for material from which it is possible to obtain a drug with a presumably antiviral activity is carried out by several research institutes.
MATERIAL AND METHODS
We used the influenza virus A/P/R/8/34 adapted to white mice (passage 4) at a dose of 20 LD/0.1 ml, titre HA (hemagglutinin) 1:32. White mice, Balb/s lines, weighing 12-14 grams. Trypsin inhibitors: Ovomukoid-isolated duck eggs from the Institute of Biochemistry named after N, I, Bach of the Russian Federation, an Inhibitor of sprouted barley grains and Trielin isolated from the submandibular glands of a dog by employees of the Agro-Industrial Institute of Selection and Genetics of the Ukrainian Academy of Sciences. Infection of animals with influenza virus and treatment with inhibitors was carried out intranasally under mild ether anesthesia. Were studied: 0.5 mg/ml; 2.5 mg/ml; 5.0 mg/ml; and 10 mg/ml. Treatment regimens differed only in the initial stages: 1 hour before infection, during infection, 1 hour after infection, 6 hours after infection, 24 hours after infection, 48 hours after infection,72 hours after infection, and 96 hours after infection. The work used virological and biochemical techniques. The trypsin-like proteinase activity was evaluated by the method of Veremeenko [11] modificated by Vovchuk [12]. The determination of proteinase inhibitors in lung homogenate and blood serum was carried out by the casein method proposed by Levitsky [13] modificated by Divocha [14]. The hemagglutination reaction was set according to the standard method. Determination of protein was performed according to the method of Lowry [15].
RESULTS
As shown by the research results, a trypsin inhibitor isolated from barley by the biochemistry laboratory of the Agro-Industrial Institute of Selection and Genetics did not have a protective effect in all doses studied. The first inhibitor preparations were also very toxic to formic acid residues (Table 1). The drug had to be further purified and freed from impurities of formic.
Table 1. The presence of hemagglutinating activity in the lungs of dead mice infected with a lethal dose of influenza virus A/P/R/8/34 and treated with a trypsin inhibitor at a dose of 2.5 mg
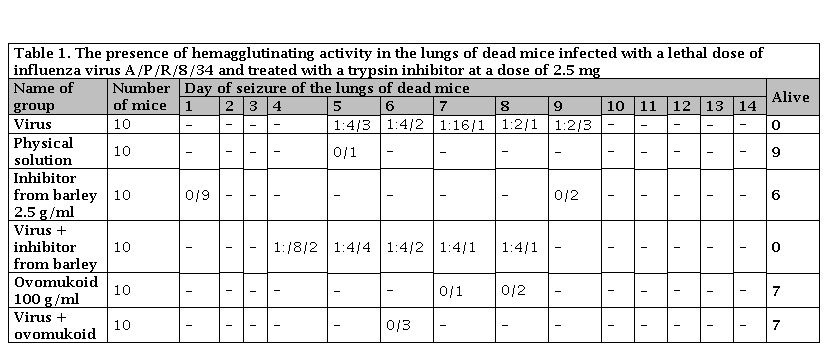
Numerator is the number of dead mice; the denominator is the number of surviving mice
Using trypsin inhibitor from barley at a dose of 10 g/l, we observed that did not protect animals from death, but delayed the development of a viral infection for 8 days (Table 2).
Table 2. The presence of hemagglutinating activity in the lungs of dead mice infected with a lethal dose of influenza virus A/P/R/8/34 and treated with trypsin inhibitors

Numerator: Titer HA; Denominator is the number of mice from which the lungs were taken
Ovomukoid inhibitor isolated from duck eggs at the Institute of Biochemistry named after I.O. Bach, Russian Academy of Sciences. We found that with the introduction of an ovomukoid at a dose of 100 gamma/ml 1 hour before infection and further according to the scheme, the development of influenza infection did not occur (Table 1). 70% of the animals remained alive until 14 days (observation period). 0% of animals, both in the experiment and in the control, died from the toxicity of the drug itself. Hemagglutinin was not found in the lungs of mice killed by influenza. When we introduced this drug to animals 1 hour after infection and then according to the schedule, we did not observe a protective effect even when the dose of the inhibitor was increased to 10 g. However, with an increase in the dose of the inhibitor, the percentage of mortality of animals from the inhibitor increased, and when using a dose of 10 mg/ml, 100% mortality of animals occurred (Table 2). Thus, the Ovomukoid possessed only a prophylactic property
Trielin inhibitor isolated from the salivary glands of a dog at the Agro-Industrial Breeding and Genetics Institute of the Academy of Sciences of Ukraine When using trielin in a dose of 500 gamm/ml and administering it after 1 hour after infection, the protective effect of its lethal influenza infection was not observed (Table 3). With an increase in the dose to 10 g/l and its administration simultaneously with the virus, the presence of hemagglutinin in the lungs of the treated mice began to be noted only on the tenth day after infection (Table 4). 40% of the animals remained alive on day 14 (time of observation). The drug was not toxic to animals.
Table 3. The effect of trypsin inhibitors at a dose of 10 mg/ml on the survival of mice infected with a lethal dose of influenza virus A/P/R/8/34
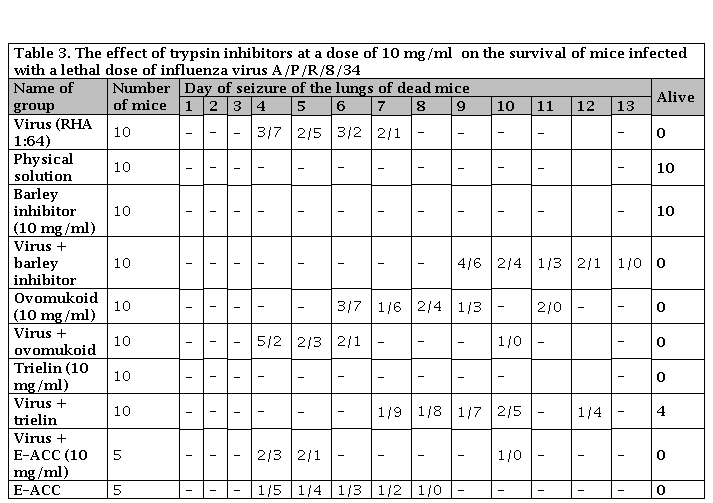
Numerator: Number of dead mice; Denominator: Number of mice in the experience
Table 4. The presence of hemagglutinating activity in the lungs of dead mice infected with a lethal dose of influenza virus A/P/R/8/34 and treated with trypsin inhibitors
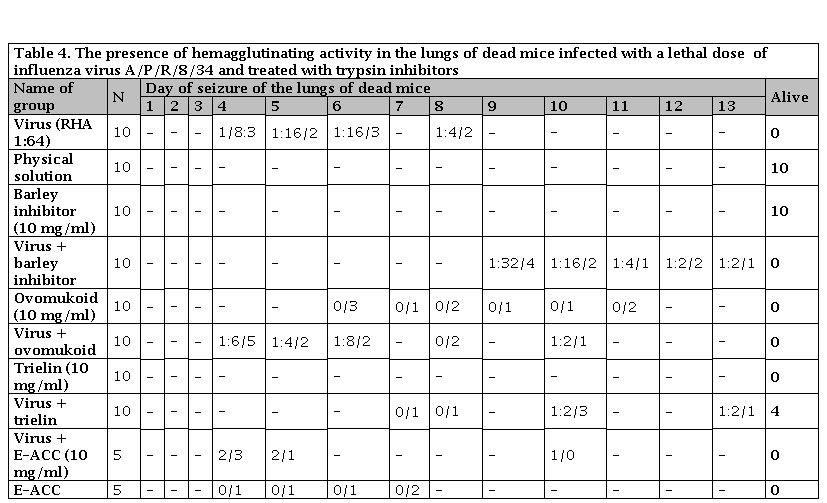
Numerator: Titer HA; Denominator is the number of mice seized from the lungs .N: Number of mice
Thus, trielin at a dose of 10 g/l when administered simultaneously with the virus, had a pronounced antiviral effect.
DISCUSSION
The proteolytic system of animals, including humans, plays a key role in the development of the pathological process in influenza [16]. Trypsin-like proteinase breaks down the influenza virus hemagglutinin external protein into two subunits HA1 and HA2 [17]. Only after cleavage of hemagglutinin with trypsin-like proteinases does the influenza virus enter the cell and begin to multiply. Our previous studies have been experimentally established that a cellular trypsin-like proteinase inhibitor blocked proteinase and the experimental viral infection did not develop - the animals remained alive even after they had been given a lethal dose of the influenza virus (18 ). Trypsin-like proteinase plays a key role in the development of the pathological process in the body. It breaks down the outer protein of the influenza virus (hemagglutinin) into two subunits - NA1 and NA2 [19]. Only after splitting the virus penetrates the cell and begins to multiply [20]. A new approach to the treatment of influenza: blocking the proteolytic activation of the virus by proteinase inhibitors is currently considered the most promising [21]. The mechanism of the antiviral effect of inhibitors is associated with suprression of proteolytic activity [22]. Data from a series of virus models indicate that the mechanism of proteolytic activation has a general biological universal value. In picornaviruses and togas viruses, proteolytic
(hemagglutinin) into
The proteolyitic system of animals, including humans, plays a key role in the development of the pathological process in inflñuenza [16]. Trypsin-like proteinase breaks down the influenza virus hemagglutinin external protein into two subunits HA1 and HA2 [17]. Only after cleavage of hemagglutinin with trypsine-like proteinases does the influenza virus enter the cell and begin to multiply. Our previous studies have been experimentally established that a cellular trypsin-like proteinase inhibitor blocked proteinase and the experimental viral infection did not develop - the animals remained alive even after they had been given a lethal dose of the influenza virus [18]. Trypsin-like proteinase plays a key role in the development of the pathological process in the body. It breaks donw the outer protein of the influenza virus two subunits - NA1and NA2 [19]. Only after splitting the virus penetrates the cell and begins to multiply [20]. A new approach to the treatment of influenza: blocking the proteolytic activation of the virus by proteinase inhibitors is currently considered the most promising [21]. The mechanism of the antiviral effect of inhibitors is associated with suppression of proteolytic activity [22]. Data from a series of virus models indicate that the mechanism of proteolytic activation has a general biological universal value. In picornaviruses and togas viruses, proteolytic activation is related to the formation of mature viral proteins. In influenza viruses and paramyxoviruses, proteolytic activation is associated with the breakdown of glycoproteins, which cause the virus to enter the cell. Inhibitors block the process of cutting viral proteins by inhibiting the activity of cellular enzymes. In the presence of cellular proteinase inhibitors, after one cycle of reproduction of the original virus with chopped proteins, viral offspring with undigested, functionally inactive viral proteins are formed. Virions are not capable to initiate an infectious process in connection with the block of the early stages of the reproduction cycle [23].
For the first time in world practice, we have isolated a trypsin-like protein inhibitor from the lungs of healthy mice. It was characterized by a high degree of purity and contained a small amount of impurities. The inhibitor had a molecular mass of 47500 Yes. Developed and patented method of obtaining and cleaning the inhibitor trypsin-like proteinases [24]. The dedicated inhibitor is similar to the alpha-1-inhibitor of human serum proteinase (m.m. 48000- 55000 Da) and an egg protein trypsin inhibitor (m.m. 49000 Da), but not similar to the trypsin inhibitor isolated from the lungs of cattle (a Kunitz-Northrop-type inhibitor) that had a molecular mass of 65000 Da. Proteolytic activity of the isophforms trypsin-like protein test method, it was found that it suppressed the activity of almost all isoforms, except the fourth (41.8%) eighth (28.3%).
In our research, using a cellular inhibitor to suppress the development of influenza virus in chicken embryos, it has been found to inhibit the development of infectious and hemagglutinating activity and the formation of a common protein. At the same time, the trypsin-like protein inhibitor, isolated from the lungs of mice pre-infected with the influenza virus, did not have this ability. In further studies, we used an inhibitor to treat influenza infection in animals, which was isolated from the lungs of healthy mice. In the treatment of white mice pre-infected with a lethal dose of influenza virus, 80% of the animals recovered and remained alive for up to 14 days after infection.
To obtain a new generation antiviral drug, many research groups received trypsin-like protease inhibitors. So, the drug Trypsin Inhibitor from barley, delayed the development of viral infection up to 8 days. We suggest that increasing the dose of the inhibitor will protect animals from death. These studies should be continued. Ovomukoid, at a dose of 100 gamm/ml, introduced 1 hour before infection, we offer for the prevention of influenza. The trielin drug turned out to be the most encouraging; when treated with it, 40% of the animals remained alive and it was not toxic to animals only. Research is ongoing.
CONCLUSIONS
Ovomukoid inhibitor, possessing prophylactic properties at a dose of 100 gamma / ml, but was toxic to animals when the dose was increased
Inhibitor Trielin, had a pronounced therapeutic effect in influenza and was not toxic.
The Barley trypsin inhibitor in the studied doses did not exert an inhibitory effect in case of influenza infection.
Our results did not exert a protective effect in the same dose and E_AKK used for influenza in some clinics in Ukraine.














