1. INTRODUCTION
Patients with end-stage liver diseases (ESLD), such as cirrhosis of different etiologies, autoimmune liver diseases, genetically linked liver malformations and other have a poor prognosis and liver transplantation (LT) is a life-saving treatment for them. Scientific studies discuss the influence of gut bacteria on normal and pathological liver function. With the advent of advanced molecular biological techniques to facilitate culture-independent characterization of microbiota, it is possible to study in details different bacteria in the human microbiome [1]. This allows us to understand their role in the clinical setting during and after LT. The connection between intestinal microbiome, immunity and its dysregulation is well established, as well as the changes that occur in the post-transplant period under the influence of immunosuppressive therapy. A number of studies have shown that changes in the microbiome may be associated with acute graft rejection, as well as other post-transplant complications. Gut microbiota modulate some systemic and immune responses via multiple mechanisms that may affect allograft function [2]. It has been found that some bacteria and their metabolites have a beneficial effect on innate and acquired immunity, reducing T-cell activation and promoting induction of regulatory T cells (Tregs) [3]. This is essential for building and maintaining graft tolerance [4].
The barrier functions of the intestinal mucosa are very sensitive to dysbiosis (condition when the gut bacteria become imbalanced) and are often disrupted which could lead the bacterial metabolites to enter the circulation [1]. Gut dysbiosis could alter the barrier functions of the mucosa; followed by reduced production of mucin, which coincides with the loss of the beneficial microorganism Akkermansia muciniphila (AKM) [5]. Faecalibacterium prausnitzii (FAEP), which is a bacterium with potent anti-inflammatory properties, via nuclear factor κB inhibition and induction of Treg [6], is also of great interest for research. It has been found that the decrease in the total amount of FAEP is accompanied by overgrowth of some opportunistic species, which increases the risk of infections in patients [7].
Many factors can affect the gut microbiota after LT, such as antibiotics, pro/prebiotics, and immunosuppressive therapy that can additionally modify the baseline gut bacterial dysbiosis present in ESLD, emphasizing the importance of understanding the impact of gut microbiota post LT [1, 8]. However, it should be noted that there are age related changes in the gut microbiota with infant microbiota being relatively volatile [9]. Calcineurin inhibitors (CNI), including tacrolimus and cyclosporine A (CsA), are the main immunosuppressive drugs used in the treatment of patients after LT [10]. Optimal dosing is extremely important for a better allograft outcome. It has been shown that both high and low doses of CNI could suppress some beneficial bacteria, such as Faecalibacterium prausnitzii [11]. Mycophenolate mofetil (MMF) is potent immunosuppressive agent used as adjunctive therapy in prevention of allograft rejection. MMF has been shown to be associated with frequent diarrhea and dysbiosis, as well as a reduction in some beneficial bacteria including AKM [10]. The mammalian target of rapamycin (mTOR) inhibitors, such as sirolimus and everolimus, have a favorable adverse event profile and are effective in protecting kidney function in LT patients. Everolimus appear to have little impact on intestinal microbiota [12].
We studied the distribution and quantities of AKM and FAEP in Bulgarian patients after LT and subjected to immunosuppressive therapy. The aim of this pilot study was to investigate the prevalence and quantity of these two bacteria in the late post-transplant period.
2. MATERIALS AND METHODS
This study was performed between May 2021 and October 2021 at the University Hospital "Lozenets", Sofia, Bulgaria. In total 32 individuals, children and adults, designed into two groups: control group (9 persons) and patients after LT (23 persons) were included in the study. Patients were enrolled according to inclusion criteria: with liver transplantation at least 3 months after the procedure and on immunosuppressive therapy; without systemic or gut infections and antibiotic usage at least two months before enrolment; no data for acute rejection; no autoimmune diseases, diabetes and carcinoma. Exclusion criteria: active hepatitis B virus, hepatitis C virus, human immunodeficiency virus infections or tuberculosis; alcohol and cigarette abuse, acute diarrhea. All patients with LT were in good general condition. Control groups (children and adults) were healthy individuals without infections and antibiotic treatment in the last 2 months.
The study was conducted following the ethical guidelines of the Declaration of Helsinki and was approved by the local ethics committee (protocol No:2/2021). All participants have declared and signed their informed consent. Parents or guardians signed the informed consent for children (< 18 years old). The liver transplantions were performed at the University Hospital "Lozenets" (Sofia), being also performed the follow-up monitoring in the post-transplant period.
2.1. STOOL SAMPLES COLLECTION
Fecal samples were obtained from children and adult patients admitted to the hospital for routine examination. Sterile stool collection tubes (Prima, 20 ml) were used; the samples were immediately transported to the laboratory and stored at - 20°C until later analysis.
2.2. ISOLATION OF BACTERIAL DNA FROM FECAL SAMPLES
Bacterial DNA was isolated from the fecal samples using the stool DNA isolation kit (QIAamp Fast DNA Stool Mini Kit, ref.51604, Qiagen, Germany), according to the manufacturer's guidelines, with a slight modification. Briefly, 1 ml InhibitEX buffer and sand particles were added to 200 mg feces; the samples were incubated at 95°C for 10 minutes, to promote better lysis of Gram-positive bacteria. Additional homogenization and lysis of the samples was done on Precellys 24 (Peqlab,Gmbh) for 15 sec. at 4500 rpm. The resulting homogenate was centrifuged (3 min at 17000 g) and samples were processed for DNA isolation. The concentration and purity of the DNA was determined spectrophotometrically using a NanoDrop 2000 spectrophotometer (Thermo Scientific, USA). The mean purity of the DNA was (mean ± SD) 1.90 ± 0.10, ratio Abs260/280 nm. The DNA samples were stored at -20°C until further testing was performed.
2.3. QUANTIFICATION OF AKKERMANSIA MUCINIPHILA AND FAECALIBACTERIUM PRAUSNITZII BY QPCR METHOD
After extraction, fecal bacterial DNA was quantified and adjusted in order to obtain DNA 12.5 ng/μl. qPCRs included 4 μl template (50 ng of DNA per reaction) and 16 μl of Reaction/Master mix. To quantify the amount of AKM and FAEP DNA we used MutaPLEX® AKM/FAEP real time PCR kit (Immundiagnostik AG, Germany), following the manufacturer's instructions. Samples were analyzed using LightCycler 480 II thermocycler (Roche). For quantification of Akkermansia muciniphila and Faecalibacterium prausnitzii positive DNA in samples, a standard curve using standards was applied. The data obtained in copies per reaction were then converted to cells/g, according to the protocol and the results are presented in log 10 of bacteria cells per g feces.
3. RESULTS
The average post-transplant period was 7 years (min-max: 2 – 17y). The indications for transplantation in adults were decompensated liver cirrhosis of various etiologies (ethylism - 4, viral - 4, autoimmune - 2 and 6 with other etiology). In children, the cause for transplantation was: autoimmune hepatitis - 1, biliary atresia - 3, liver cirrhosis of unknown etiology - 4. Control groups included healthy individuals without LT. Gender and age of the subjects groups are presented in Table 1.
Table 1: Demographic features of patients and control groups.
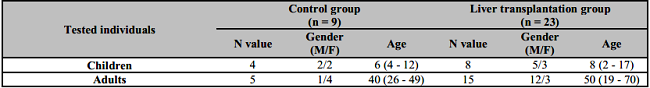
M: Male; F: Female. Age expressed as mean as well as minimum and maximum values.
We studied the presence and amounts of AKM and FAEP in fecal samples of all enrolled persons. The results are shown on Table 2. We found that in the control group, all individuals had FAEP and AKM and only one lacked AKM. In patients with LT group, however, we found that FAEP was observed in 83% and AKM in only 52% of transplant recipients. These differences were statistically significant when the amounts of bacterial expression was compared for FAEP and АКМ, respectively p = 0.003 and p = 0.03. AKM was not detected in 48% of the patients and FAEP in 17% of the patients.
Table 2: Prevalence and amounts of FAEP and AKM in fecal samples of control and liver transplanted patients.

*Mann-Whitney Exact U Test was used to compare the amounts of the two bacteria. p<0.05. FAEP: Faecalibacterium prausnitzii; AKM: Akkermansia muciniphila.
The separate analysis in Children and Adults groups shows that this trend is more pronounced in children than in adult transplant subjects. In all studied children (controls and patients with LT) we found the presence of FAEP, but the measured amounts in the transplanted group were significantly lower than healthy controls (p = 0.014, Table 3). The analysis of AKM in children found that 3 out of 8 children (38%) had AKM in the fecal samples, eg. 62% of children with LT do not have AKM expression in their feces. The statistical comparison between the control and LT patient groups revealed reduced amounts of AKM in patients (p = 0.051, Table 3).
Table 3: Distribution and quantity of FAEP and AKM in children control and liver transplantation groups.

*Mann-Whitney Exact U Test was used to compare the amounts of the two bacteria. p<0.05. FAEP: Faecalibacterium prausnitzii; AKM: Akkermansia muciniphila.
The results of the research in adults are presented in Table 4. The frequency of positive FAEP samples is 100% in the control group, while in the transplanted patients it is 73%. In 27% of patients with LT, this bacterium was absent. AKM expression was also reduced in patients with LT - 67% versus 80% in the control group. Notably, in 33% of transplanted patients AKM were not detected. The median amounts for both bacteria in patients were reduced compared to healthy controls, but the differences were not statistically significant (Table 4).
Table 4: Distribution and quantity of FAEP and AKM in adult control and liver transplantation groups.
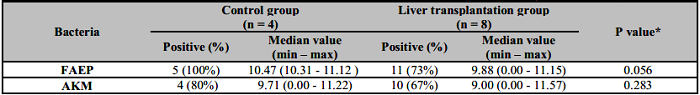
*Mann-Whitney Exact U Test was used to compare the amounts of the two bacteria. p<0.05. FAEP: Faecalibacterium prausnitzii; AKM: Akkermansia muciniphila.
One possible reason for the decreased bacterial expression in the gut of LT patients might be the immunosuppressive therapy of these patients. Seven adult patients were treated with tacrolimus and MMF, four - received tacrolimus alone, one patient was on everolimus and cyclosporine, and one on cyclosporine and MMF. Seven of the children with LT were treated with tacrolimus and one child (autoimmune hepatitis) was on cyclosporine, methylprednisolone and MMF. Our results concerning the reduced prevalence of both bacteria in LT patients, subjected to different immunosuppressive regimens, are shown on Table 5.
Table 5: Patients with liver transplantation with no detection of AKM or/and FAEP in context of immunosuppresive regimens.
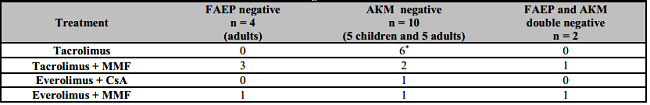
*5 children + 1 adult patients. FAEP: Faecalibacterium prausnitzii; AKM: Akkermansia muciniphila; MMF: Mycophenolate mofetil; CsA: Cyclosporine A.
The following conclusions can be drawn from Table 5:
FAEP was detected in all children fecal samples. In contrast, the prevalence of AKM in both patient groups was lower in comparison with controls. Furthermore, two adult patients were found to be double negative.
It should be noted that all regimens of used immunosuppressive therapy most likely influence AKM growth, as it was not detected in 10 out of 23 patients with LT (43%). We can assume that the calcineurine inhibitor tacrolimus might suppress both studied bacteria, but apparently its combination with MMF enhances this effect.
The two double-negative patients (lacking both FAEP and AKM) are on therapy with MMF and tacrolimus or everolimus.
4. DISCUSSION
Akkermansia muciniphila andFaecalibacterium prausnitzii are two commensal bacteria, symbiotic and numerically abundant members of the gut microbiota. Recent studies have demonstrated their possible association with dysbiosis in several disease conditions [13]. Moreover, both species are now shown to have a role in a well-functioning gut and thus are considered as promising next generation probiotics [14, 15].
In our study, we found a reduction in the prevalence and amount of AKM and FAEP in patients in the late post-transplant period compared to the control healthy group. This was more markedly seen in children with LT, where AKM was not detected in 62% of the studied samples. The limitations of the current study include small number of patients and control individuals, single testing of all participants, single-center design, the COVID 19 pandemic, which further complicates the enrolment of immunosuppressed patients.
Recently, it has been shown that after LT there is a decrease in gut bacterial diversity and dysbiosis [1, 8]. It has been found that changes in gut microbial composition can result in disruption of the mucosal barrier, facilitating the translocation of bacteria and microbial products pathogen associated molecular patterns in the portal circulation affecting the inflammatory cytokine milieu in the liver [16].
The pre- and post-LT comparative analysis observed a decrease in gut microbial diversity in the early post-transplant period (1 month), with improvement in diversity after at least 6 months [17]. Furthermore, in a qPCR-based study of 111 LT recipients it was found that the amount of FAEP was significantly reduced in recipients, which corresponds with our results [7]. Faecalibacterium prausnitzii is an anaerobe with a fecal-mucosal distribution, one of the major producers of SCFAs (short-chain fatty acids) of which butyrate is the main energy source for colonic epithelium and possesses potent anti-inflammatory properties [18]. This bacterium is considered as an anti-inflammatory with an essential role for the maintenance of the colonic mucosa, the induction of regulatory T cells [18] and the regulation of Treg/Th17 balance [19].
Our data shows a significant reduction of AKM load in LT patients. In the literature, there are discrepant data. Satapathy SK et al. found loss of AKM in patients after LT, especially in patients with recurrent NAFLD. It has been suggested that AKM may play a protective role in the development of complications (such as de novo NAFLD) after LT [20]. However, Sun et al., found an increase in potentially beneficial bacteria, such as AKM, Blautia and Clostridiales cluster XIVa, 3 months after LT. The authors suggested that the transplanted liver could significantly improve gut function, hence leading to an increase in beneficial bacteria in the fecal microbiota [21]. A. muciniphila is an anaerobic, mucin-degrading bacterium [15], which is considered to have a protective role in the barrier function of the intestinal mucosa by strengthening the contacts between intestinal epithelium cells (tight junctions). Furthermore, it has been suggested that a decrease in the total amount of AKM can be associated with thinning of the mucin layer, which may contribute to liver inflammation [5].
In addition, we try to analyze our data in the context of the applied immunosuppressive therapy. The results showed that AKM is mostly affected by tacrolimus and the combination of tacrolimus with MMF. The available in literature data on this topic is limited and there is unequivocal opinion on how tacrolimus affects AKM. Most studies have been performed on mouse models of LT and immunosuppressive treatment. Same as us, some authors indicate a reduction in the load of this bacterium after tacrolimus treatment [22], in contrast others report that tacrolimus increases the amount of AKM [10]. It has been found in mice that the medium dose of tacrolimus increased the amount of FAEP, while both low and high dose of immunosuppressant reduced the bacteria [11]. Concerning FAEP, we found a reduction in the bacterial load after combined immunosuppression of tacrolimus/everolimus and MMF, whereas patients in tacrolimus only do not showed lack of F. prausnitzii. Intriguingly, it has been observed that in patients who require higher drug doses to reach optimal tacrolimus plasma concentrations, the amount of FAEP is increased [23]. The authors found that some Clostridiales including FAEP could transform tacrolimus into a less potent metabolite. The aforementioned study provides evidence for new pathways of tacrolimus metabolism and the role of the gut microbiota in it.
5. CONCLUSIONS
Our pilot study shows that in patients with LT on immunosuppressive therapy in the late post-transplant period, the frequency and amount of beneficial bacteria AKM and FAEP in gut microbiota are significantly reduced in comparison with healthy individuals. This may be due to a variety of possible factors, one of which is immunosuppressive therapy. Studies in a larger number of patients are needed for confirmation of our results and further analysis.














