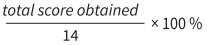Highlights
Polycystic ovary syndrome (PCOS) affects a significant percentage of women in their reproductive age and can lead to various health issues such as infertility, hirsutism, menstrual irregularities, hyperandrogenism, insulin resistance, abnormal glucose tolerance, and high risk for type 2 diabetes mellitus.
Obesity and insulin resistance are common in PCOS patients, and managing metabolic and hormonal functions can help manage PCOS symptoms. Losing weight can also improve symptoms, but it can be challenging.
Blood tests and ultrasound can help diagnose PCOS, but it is a difficult syndrome to diagnose. Metformin and exenatide are being studied as potential treatments, and a meta-analysis is required to review and draw conclusions on their efficacy in controlling symptoms, metabolic status, and hormonal state in PCOS patients.
Introduction
Polycystic ovary syndrome (PCOS) affects 6-15% of women in their reproductive age making them infertile, hirsutisms, menstrual irregularities, hyperandrogenism, polycystic ovaries, insulin resistance (IR), abnormal glucose tolerance and high risk for type 2 diabetes mellitus. Obese patients and 30% of normal-weight PCOS patients manifest insulin resistance. Insulin resistance results in hyperinsulinemia in patients, thus disturbing the normal function of the ovaries and ovulation rate. It causes the ovaries to produce higher amounts of androgen than the normal range.(1, 2) Women with PCOS then develop acne in their faces and experience severe hair fall and thinning of scalp making them bald but not as severe as male baldness.3 PCOS patients are also at risk of developing serious health issues like diabetes, heart disease, high blood pressure, high LDL cholesterol, low HDL cholesterol.4 The real cause of PCOS is still unidentified but there are some possible causes that lead to PCOS which are hereditary. Genes that are passed down from their parents or even passed down from their ancestors leads to PCOS in women.3 Research has shown that the PCOS patients who is obese and manifest insulin resistance have increased number of white blood cells. Obese and insulin resistance leads to low-grade- inflammation which proves for the elevation of white blood cell in PCOS patients who are obese and have insulin.5 Blood tests are also very helpful to diagnose PCOS as they can reveal our hormone status in the body, which helps the diagnosis more. Additionally, ultrasound can be used to scan the condition of the ovaries and uterus. PCOS is a condition that is difficult to diagnose, but doctors can examine women with it by performing a pelvic examination to see if there are any abnormalities.3 Over the years, many interventions to cure PCOS or to reduce the symptoms have been initiated. But not all the interventions are working effectively in helping the PCOS patients.6 Researchers concentrate on managing patients’ metabolic and hormonal functions because PCOS is a difficult syndrome to treat and manage.7) Obesity and insulin resistance are the most common side effects of PCOS. They find losing weight a big challenge or even experience excessive weight gain at a very short period. Therefore, to handle and control the metabolic complications an accurate and effective intervention must be studied and investigated. Also, the effectiveness of exenatide in improving insulin resistance in PCOS patients has not been proven.8) In addition, PCOS patients are finding it hard to manage the symptom because there is no effective treatment for the condition.9 Additionally, individuals with PCOS exhibit insulin resistance, which is the primary aetiology for pre-diabetics in the general population.10 Therefore, metformin and exenatide are expected to be effective in managing the symptoms of PCOS but the outcome of the drugs is still unclear.6)
Furthermore, the effectiveness of exenatide drugs on obesity and diabetes have been studied but the efficacy on the ovaries and uterus have not yet been investigated enough. Moreover, the effectiveness of exenatide on the insulin level, ovary, and uterus when the patient is in stress are also not studied in detail. Stress is also another factor that disturbs the normal hormonal function and causes infertility.7 Meta-analysis is a study done to quantitatively assess the validity and form a conclusion based on the previous research done on the topic of interest. Meta-analysis is a subset of a systematic review. The outcome from the meta-analysis can provide an accurate result of the effect of a treatment or the risk a disease possesses.11) Therefore, the data from meta-analysis aid in estimating the outcome in PCOS patients upon using the drugs Metformin and Exenatide. Meta-analysis is a systemic approach that could provide an accurate estimation of the changes in PCOS patients after being prescribed Metformin and Exenatide which was taken from individual studies.12 As insulin resistance among PCOS patients increases over time, it mostly causes more severe problems like cardiovascular disease, obesity, and diabetes. Therefore, more studies are being conducted to overcome this syndrome effectively.1 PCOS patients report higher conception rates after losing weight while obese patients experience improved symptoms after losing 5% of the initial weight. However, the effectiveness of metformin in managing weight is not satisfactory. Therefore, a study on alternative intervention and combined intervention is done to check on the effectiveness of weight management.13 Numerous studies have been conducted, but fewer have examined the effectiveness of exenatide and metformin in PCOS patients. Thus, a meta-analysis on the effectiveness of metformin and exenatide in PCOS patients is required to review and draw conclusions from the research conducted on the efficacy of both medications in controlling symptoms, metabolic status, and hormonal state in PCOS patients. Meta-analysis will provide a complete summary of all the studies done in a standardized manner to ease the process and access for future research.
Methods
The study process that involves meta-analysis is in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guideline. PRISMA 2020 has been designed for a systemic review of studies that focus on the effect of health intervention. PRISMA 2020 is also applicable to both quantitative and qualitative studies. Also, PRISMA guidelines are used in order to produce perfect and accurate reporting of the results which it directly aids in evidence-based decision-making via the guidelines of the protocol. (14
Eligibility Criteria
Eligibility criteria were determined using PICOS which is population, intervention, comparator, outcome, and study design (15 as indicated in Table 1.
Table 1. Inclusion and Exclusion criteria of the study.
| PICOS elements | Inclusion criteria | Exclusion criteria |
|---|---|---|
| Population | Women suspected and diagnosed with PCOS | Not PCOS patients |
| Irrelevant to the objective of the study | ||
| Intervention | Metformin and exenatide drug intervention for PCOS patients | Intervention that are not related to either metformin or exenatide |
| Comparator | Studies that reported the improvements of symptoms in PCOS patients under either one of the drugs, Metformin and Exenatide. | |
| Outcome | The efficacy of either one drugs or both the drugs, Metformin and Exenatide in treating PCOS patients | Non-clear data on the efficacy of the drugs |
| Study design | Focus only on research article or journals from 2017-2021 | Non-full text articles |
| Non-English articles | ||
| Irrelevant to metformin or exenatide medication |
Search Strategy and Data Source
Detailed and extensive search in five databases; ScienceDirect, Scopus, Medline, Oxford Journal, and Wiley Online Library was done on the efficacy of the drugs, metformin, and exenatide in PCOS patients. The keywords used to search research articles are PCOS, metformin, exenatide, and efficacy. Boolean operator ‘AND’ is used to connect all the keywords together to perform an article search. The publication year was restricted from 2017-2021. Research articles are only focused on the process of article search.
Scopus
(TITLE-ABS-KEY (PCOS) AND TITLE-ABS-KEY (metformin) AND TITLE-ABS-KEY (efficacy)) (TITLE-ABS-KEY (PCOS) AND TITLE-ABS-KEY (exenatide))
Wiley Online Library
“PCOS” anywhere and “metformin” anywhere and “efficacy” anywhere
“PCOS” anywhere and “exenatide” anywhere
Search Selection
Duplicated articles were removed, and the first screening was done where abstract and title screening was done. The screening was done independently, according to the inclusion and exclusion criteria by 2 reviewers. Studies that were selected in the first screening were then subjected to full text screening to ascertain it fulfilled the objective of the study. Studies that did not fulfill the criteria were excluded. PRISMA flow diagram was used to state the flow of the selection process of the study.
Data Extraction
The details of data collected for human trials included (i) human sample (ii) blood sample (iii) body measurements (iv) number of patients (v) gender (vi) age (vii) location (viii) drugs (xi) dosage (x) human ethics (xi) outcome of the study (xii) adverse symptoms and the details of animal study includes (i) organism (ii) sex (iii) weight (iv) animal sample (v) blood sample (vi) body measurements (vii) age (viii) mode of administration (xi) animal ethics (x) location (xi) type of assay (xii) drugs (xiii) dosage.
Risk of Bias in Individual Studies
Risk of bias (RoB) or the quality assessment is done to make sure the data that is extracted from the studies are transparent and the results that are produced are valid. The risk of bias for the human trials and in vivo study would be different.
Human Trials RoB tools
The risk of bias assessment for the human study16 was done in the RevMan software. There were 6 selection biases. They were selection bias, performance bias, detection bias, attrition bias, reporting bias, and other biases. The risk of bias and state of the assessment were evaluated in 3 ways. They were high risk, low risk, and unclear risk.
In-vivo RoB tools
The Systematic Review Center for Laboratory animal Experimentation (SYRCLE) RoB tool will be used for the risk of bias assessment for the in-vitro study. The (SYRCLE) RoB tool has 10 entries and that entry has 6 types of bias which are selection bias, performance bias, detection bias, attrition bias, reporting bias and other biases. There are some signaling questions that must be answered with a yes, unclear, or no in order to undertake the risk of bias assessment. If the answer was “Yes”, it indicates a low risk of bias. If the answer was “no”, it indicates high risk of bias. If the answer was “unclear”, it indicates an unclear risk of bias.17
Data Analysis and Result Analysis
The data that were extracted and analyzed in RevMan to obtain the pool result. The overall result was acquired after adjusting the effect model which was the fixed- effect model or random- effect model. In order to choose between fixed effect model and random effect model the I2 Statistics is crucial.18
Reported that statistics of 50-90 % portrays a substantial heterogeneity. If the I2 statistics is less than 50% then the heterogeneity is not significant therefore a fixed effect model would be used. If, the I2 statistics is more than 50% then the heterogeneity is significant therefore a random effect model would be used. The results of 95% CI were also recorded. In the forest plot, the lines that indicate the 95% CI specify the significance of the result. Therefore, if the line passes zero then the result is known to be non-significant.
Results
The article search was done precisely and extensively by using 4 keywords which were (PCOS AND metformin AND efficacy) (PCOS AND exenatide). A total of 965 articles were selected from 5 databases (ScienceDirect, Scopus, Wiley Online Library, Oxford Journal Online, PubMed). A total of 389 articles from ScienceDirect, 155 articles from Scopus, 234 articles from Wiley Online Library, 100 articles from Oxford Journal Online and 87 articles from PubMed. A total of 65 duplicated articles were removed.
In the first screening, the title and abstract were screened, a total of 762 articles were removed because they were not according to the inclusion criteria. After the first screening, 138 articles remained. The 138 articles were subjected to full text screening. After full text-screening, 58 articles were included and 80 articles that do not fulfill the criteria were removed. The 58 articles has full text available online, are original articles which focus on PCOS, are published in English language, are not meta-analysis nor systematic review type of article papers, do not discuss on metformin and exenatide efficacy and have sufficient data to be discussed and Only 6 articles were included for meta-analysis as they have discussion on metformin and exenatide efficacy and have sufficient data to be discussed (Figure 1).
Characteristics of the Study Selection
There were two types of subjects used: human and rats/mice. In human trials, there were 4 types of drugs that were used to study among PCOS women which were metformin monotherapy (Table 2), exenatide monotherapy (Table 3), metformin and exenatide combination therapy (Table 4) and other combination drug treatment (Table 5). In human trials, blood samples and body measurement were taken to measure Low Density Lipoprotein (LDL), High Density Lipoprotein (HDL), testosterone and Body Mass Index (BMI). The age of PCOS patients were between 18 to 40 years old. Each study did not exceed 40 years of age because after the age of 40, females may start having period irregularities and reach the stage of menopause. In in-vivo study, there were combination of drugs that were used to study among rats and mice (Table 6). Sprague Dawley rats were more commonly used followed by Wistar rat and BALB C mice. The mode of drug administration was either through oral, subcutaneous, and intraperitoneal routes. Blood test result and body measurement were used to observe the drug effect. The total number of papers that were excluded from the study selection was 80; 19 articles had no full articles access, 11 were non-original articles, 5 were non-English and non-PCOS articles, 39 were review, systemic review, and meta-analysis articles, 1 article was not related to efficacy of metformin and exenatide and 5 articles had no adequate data.
Table 2. Summary of metformin treatment in human trials.
| Outcome of the study | References |
|---|---|
| Larger head circumference of the newborns in metformin group. | (19) |
| Reduced acne and hirsutism. The abortion rate is also reduced, and menstrual cycle were regulated. | (20) |
| Loss of body mass, menstrual frequency increased, and testosterone and androstenedione level decreased. | (21) |
| Reduced BMI and BP, reduce in the waist hip ratio level and increase in LDL size and adiponectin. | (22) |
| The BMI of the patients were reduced, improved menstrual cycle, and decreased level of testosterone, and luteinizing hormone. | (23) |
| Ovulation was successful for around 84% of women. | (24) |
| Improved the BMI and insulin resistance of PCOS patients. | (25) |
| Reduced body weight and improved menstrual cycle. | (26) |
| Testosterone level dropped. | (27) |
| Effective in ovarian hyperandrogenism and acne in women with PCOS. | (28) |
| Improves peripheral endothelial function in PCOS. | (29) |
Table 4. Comparison of metformin and exenatide treatment in human trials.
| Outcome of the study | References |
|---|---|
| Exenatide showed better loss of weight, improving insulin resistance and reducing inflammation than metformin. Metformin showed reduced level of total cholesterol as compared to exenatide. | (1) |
| LDL levels declined in Metformin; HDL lipid levels remained the same in all treatment group. | (10) |
| Exenatide is more effective in regulating the menstrual cycle then Metformin. Testosterone level reduced in both treatments. BMI was reduced more in exenatide group than the Met group. | (6) |
| COM therapy was more effective than Metformin alone in reducing body weight, BMI, and improving insulin sensitivity in overweight or obese PCOS women. | (13) |
| A significant increase in circulating ZAG. | (32) |
Table 5. Summary of other combination drug treatment in human trials.
| Drugs | Outcome of the study | References |
|---|---|---|
| Metformin and clomiphene | Follicular development and ovulation rate increased | (33) |
| Metformin and saxagliptin | Improved insulin sensitivity, metformin and combine drugs showed a reduced in total cholesterol level, metformin shows decrease in the LDL-C and increase in HDL-C levels | (34) |
| Cinnamon, metformin and darchini | Testosterone level was maintained in patients with both cinnamon and metformin and change in ovarian size was observed. | (35) |
| N-acetyl cysteine, metformin, and clomiphene citrate | Serum concentrations of total testosterone and fasting insulin was reduced in metformin and BMI was reduced too. | (36) |
| Sitagliptin and metformin | Metformin group experienced a most reduced in body weight and improved the menstrual cycle | (37) |
| Metformin, inositol | The M1 oocyte count was significantly lower in the metformin group than in the inositol and control groups | (38) |
| Exenatide, dapagliflozin, or phentermine/ topiramate and metformin | EQW/DAPA and PHEN/TPM showed better weight loss, reduced in waist circumference and waist to hip ratio compared to DAPA and DAPA/MET | (39) |
| Metformin and myoinositol | Metformin reduced the body weight and improved menstrual cycle. | (40) |
| Metformin and oral contraceptive pills | Metformin and OCP reduced body weight and increase in triglecerides | (41) |
| Metformin and empagliflozin | The empagliflozin showed a decrease in BMI, WC and total fat mass as compared to metformin which were all increased. | (42) |
| Combined oral contraceptive, spironolactone and metformin | The hirsutism score, serum total testosterone, free testosterone and androstenedione concentrations decreased more with COC plus spironolactone than with metformin. The HDL and TG were also increased in the COC plus spironolactone than with metformin. | (43) |
| Bushen Huatan Decoction and metformin | HOMA-IR of Bushen Huatan Decoction combined with metformin group will decrease more than metformin alone. | (44) |
| Metformin and cabergoline | The use of cabergoline and metformin had improvement on cycle regularity and reduced level of prolactin in PCOS than the use of metformin alone. | (45) |
| Metformin, myoinositol and plus D-chiro inositol | Combined therapy with metformin and MI plus DCI in women with PCOS and insulin resistance shows improvement | (46) |
| Metformin and N-acetyl cysteine | NAC found to improves some clinical features, biochemical markers of insulin resistance and hormonal levels. | (47) |
| Metformin and isotretinoin | Metformin is effective in treating acne and improving metabolic status. Metformin shows better improvement in PCOS patients with acne as compared isotretinoin. | (48) |
| Metformin and placebo | Metformin reduced number of patients with late miscarriage and preterm birth. | (49) |
| Metformin and GnRH agonists | Metformin group the endometrial thickness was prominent as compared to the control group. | (50) |
| Metformin XR, saxagliptin | Saxagliptin or metformin XR administration, had improvements in central adiposity, insulin levels, glucose control, androgen levels, and menstrual cyclicity. | (51) |
| Rosiglitazone, spironolactone, and metformin | Spironolactone with rosiglitazone improves the anti-androgenic and reduces its fluid retaining effect. Metformin with rosiglitazone improves insulin sensitivity, improves anthropometric parameters without increasing adverse event rate. | (52) |
| Spironolactone, metformin, pioglitazone | Metformin with vitamin D reduces the serum testosterone in PCOS patients. Pioglitazone was observed to have reduce in body weight. | (53) |
| Exenatide injection, clomiphene citrate capsules, metformin hydrochloride tablets | Exenatide shows decreased level of LH and T in PCOS patients. Exenatide and clomiphene citrate is effective reducing the level of insulin resistance in patients and also promote ovulation. | (54) |
| Exenatide injection | Changes in weight, endothelial function, serum endothelial markers, change in inflammation (hsCRP). | (30) |
Table 6. Summary of various drug treatments in in vivo study.
| Drugs | Outcome | Reference |
|---|---|---|
| Letrozole, metformin, sodium selenite | Testosterone level was reduced in both selenium and metformin, LDL-C reduced while HDL-C was increased. | (55) |
| Metformin and catechins | Metformin or Catechins treatment reduces uterine weight, hormone secretion and insulin resistance in mice. | (56) |
| Letrozole, metformin and exenatide | Metformin and exenatide could reduce body weight, reduce serum insulin levels, and improve IR. Exenatide administration could also reduce serum FG levels. | (12) |
| Metformin, auraptene and dehydroepiandrosterone | AUR improves the maturation and IVF potential of PCOS oocytes via its anti-inflammatory property, an increase in intracellular GSH level, and protection against ROS activity. | (57) |
| Estradiol valerate, metformin and chamomile flowers | Chamomile extract works well by lowering the testosterone-level, and antiapoptotic mechanisms. Serum concentrations of urea and BUN may increase with Met therapy. | (58) |
| Ocimum kilimandscharicum, letrozole and metformin | The treatment improved regular estrous cycle restoration, improved estrogen and progesterone levels and reduced testosterone levels. | (59) |
| Metformin, pioglitazone and letrzole | Metformin and pioglitazone treatment improves both metabolic and reproductive parameters of PCOS in rats. The treatment down-regulated LH receptor mRNA expression and up-regulation of FSH receptor mRNA expression respectively in PCOS rats. | (60) |
| Letrozole, Metformin and Liuwei Dihuang Pills (LWDH) | LWDH Pills successfully normalized blood glucose level and manage the hyperandrogenemia. | (61) |
| Metformin, dapsone and testosterone enanthate | Metformin significantly improved the hormone imbalances. Metformin and dapsone markedly reduced the LDL and LDL/HDL ratio. | (62) |
| Metformin and sorafenib | The treatment exerted a synergistic therapeutic effect on PCOS-induced EH both in vivo and in vitro by regulating the process of autophagy. | (63) |
| Metformin and pioglitazone | The treatment reduced the testosterone levels in a PCOS model. | (64) |
| Sitagliptin and metformin | Combination therapy improves PCOS with IR, through upregulation of lncRNA H19. | (65) |
| Matricaria chamomilla and metformin | The treatment improved the thyroid hypofunction in PCOS rats. | (66) |
| Astaxanthin and metformin | The treatment reduced oxidative stress factor H2O2 and apoptosis activity. It increases the level of AKT expression. Ovulation was also induced. | (67) |
| Diane-35 and metformin | Diane-35 and metformin reduced body weight and testosterone level. | (68) |
| Exendin- 4 Dehydroepiandrosterone | Exendin-4 reduced weight, fasting blood glucose, fasting blood insulin, HOMA-IR and LH/FSH levels in PCOS patients. The number of follicles were increased. | (7) |
| Metformin and exenatide | Both metformin and exenatide can improve the reproductive and endocrine functions of rats with PCOS via the AMPKα-SIRT1 pathway. | (8) |
Risk of Bias
Risk of Bias was done on selected articles using the checklist from RevMan software for human studies16 while for in vivo study, Systematic Review Centre for Laboratory Animal Experimentation (SYRCLE) was used to evaluate the selected studies17 for the study on the efficacy of metformin and exenatide on PCOS patients. The percentage of the bias assessment risk was calculated where the total score of all relevant items were divided by the total possible score. The total possible score was calculated by multiplying the items with the value of two (2). This is because 2 indicates yes (low risk), 1 indicates unclear (unclear risk) and 0 indicates no (high risk).
If the percentage is more than 50 % then, the selected articles are of high quality while if the percentage is less than 50 % then, the selected articles are of low quality. The percentage of the bias assessment risk for human studies ranged from 28.57 % to 100 %. Forty-one human studies were selected. Three studies showed a high risk of bias. A study by21 showed high risk of bias in selection, performance and other bias because there was a lack of randomization, no blinding during period of study and also the process of aging in participants interfere with the observation of the study. A study by24 showed the high risk of bias in selection, performance, detection, and other bias because the participants were not randomized, not blinded and also this study was a retrospective cohort. A study by30 showed high risk of bias in selection, performance and detection bias because it was an open-label study where both the physicians and participants are aware of the intervention being prescribed. The remaining 38 articles showed low risk of bias. The percentage of the bias assessment risk for the in vivo studies ranged from 42.86% to 100%. Seventeen in vivo studies were selected and four studies showed high risk of bias. The studies showed high risk of bias in selection, performance, and detection bias because the animals were not randomized during the period of study. The remaining 13 articles showed low risk of bias.
Table 7. Risk of Bias of Human Studies.
| Reference | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Total RoB (%) |
|---|---|---|---|---|---|---|---|---|
| (19) | 0 | 1 | 2 | 0 | 0 | 2 | 2 | 50% |
| (20) | 1 | 1 | 1 | 1 | 1 | 2 | 2 | 64.29% |
| (21) | 0 | 1 | 0 | 1 | 1 | 2 | 0 | 35.70% |
| (22) | 1 | 1 | 1 | 1 | 2 | 2 | 2 | 71.43% |
| (33) | 1 | 1 | 1 | 1 | 2 | 2 | 2 | 71.43% |
| (34) | 2 | 2 | 1 | 1 | 2 | 2 | 2 | 85.71% |
| (23) | 1 | 1 | 1 | 1 | 2 | 2 | 2 | 71.43% |
| (24) | 0 | 0 | 0 | 0 | 2 | 2 | 0 | 28.57% |
| (35) | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 85.71% |
| (36) | 1 | 1 | 1 | 1 | 2 | 2 | 2 | 71.43% |
| (37) | 2 | 1 | 1 | 1 | 2 | 2 | 2 | 78.57% |
| (25) | 2 | 1 | 1 | 1 | 2 | 2 | 2 | 78.57% |
| (26) | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 100% |
| (38) | 1 | 1 | 1 | 1 | 2 | 2 | 2 | 71.43% |
| (1) | 2 | 2 | 1 | 1 | 2 | 2 | 2 | 85.71% |
| (39) | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 100% |
| (10) | 2 | 2 | 1 | 1 | 2 | 2 | 0 | 71.43% |
| (6) | 2 | 2 | 1 | 1 | 2 | 2 | 2 | 85.71% |
| (55) | 2 | 2 | 1 | 0 | 2 | 2 | 2 | 78.57% |
| (13) | 2 | 2 | 1 | 1 | 2 | 2 | 2 | 85.71% |
| (32) | 2 | 2 | 1 | 1 | 2 | 2 | 2 | 85.71% |
| (40) | 2 | 0 | 1 | 1 | 2 | 2 | 2 | 71.43% |
| (41) | 2 | 0 | 1 | 1 | 2 | 2 | 2 | 71.43% |
| (42) | 2 | 0 | 1 | 1 | 2 | 2 | 2 | 71.43% |
| (43) | 2 | 2 | 0 | 0 | 2 | 2 | 2 | 71.43% |
| (44) | 2 | 2 | 1 | 1 | 2 | 2 | 2 | 85.71% |
| (27) | 0 | 0 | 1 | 1 | 2 | 2 | 2 | 57.14% |
| (45) | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 100% |
| (28) | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 100% |
| (46) | 2 | 1 | 0 | 0 | 2 | 2 | 0 | 50% |
| (47) | 2 | 1 | 1 | 1 | 2 | 2 | 0 | 64.29% |
| (48) | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 100% |
| (49) | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 100% |
| (50) | 2 | 1 | 1 | 2 | 2 | 2 | 2 | 85.71% |
| (29) | 2 | 1 | 1 | 1 | 2 | 2 | 2 | 78.57% |
| (51) | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 85.71% |
| (69) | 2 | 1 | 1 | 1 | 2 | 2 | 0 | 64.29% |
| (52) | 0 | 1 | 1 | 1 | 0 | 2 | 2 | 50% |
| (53) | 2 | 1 | 1 | 1 | 2 | 2 | 2 | 78.57% |
| (30) | 0 | 0 | 0 | 0 | 2 | 2 | 2 | 42.86% |
| (31) | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 100% |
Q1: Random sequence generation (selection bias): Was the allocation sequence generated appropriately?
Q2: Allocation concealment (selection bias): Were the physicians unaware of allocation code up to actual patient enrolment?
Q3: Blinding of personnel/ care providers (performance bias): Were the participants, unaware of actual treatment?
Q4: Blinding of outcome assessor (detection bias): Were the outcome assessors, unaware of actual treatment?
Q5: Incomplete outcome data (attrition bias): Were all data analyzed, minimizing the impact of loses to follow up?
Q6: Selective Reporting (reporting bias): Were all relevant outcomes thoroughly reported?
Q7: Other Bias: Was the risk of any other bias low?
Table 8. Risk of bias for in vivo studies.
| Reference | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Total RoB (%) |
|---|---|---|---|---|---|---|---|---|
| (55) | 0 | 0 | 0 | 1 | 2 | 2 | 2 | 50% |
| (56) | 2 | 1 | 2 | 1 | 2 | 2 | 2 | 85.71% |
| (12) | 2 | 1 | 2 | 1 | 2 | 2 | 2 | 85.71% |
| (57) | 2 | 1 | 2 | 1 | 2 | 2 | 2 | 85.71% |
| (58) | 0 | 1 | 0 | 1 | 2 | 2 | 2 | 57.14% |
| (59) | 0 | 0 | 0 | 0 | 2 | 2 | 2 | 42.86% |
| (60) | 2 | 1 | 2 | 1 | 2 | 2 | 2 | 85.71% |
| (61) | 0 | 0 | 0 | 0 | 2 | 2 | 2 | 42.86% |
| (62) | 2 | 1 | 2 | 1 | 2 | 2 | 2 | 85.71% |
| (63) | 0 | 0 | 0 | 0 | 2 | 2 | 2 | 42.86% |
| (64) | 2 | 1 | 2 | 1 | 2 | 2 | 2 | 85.71% |
| (65) | 0 | 0 | 0 | 0 | 2 | 2 | 2 | 42.86% |
| (66) | 2 | 1 | 2 | 1 | 2 | 2 | 2 | 85.71% |
| (7) | 2 | 0 | 2 | 1 | 2 | 2 | 2 | 78.57% |
| (8) | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 100% |
| (67) | 2 | 1 | 1 | 2 | 1 | 2 | 2 | 78.57% |
Q1: Random sequence generation (selection bias): Was the allocation sequence adequately generated and applied?
Q2: Allocation concealment (selection bias): Was the allocation adequately concealed?
Q3: Random housing (performance bias): Were the animals randomly housed during the experiment?
Q4: Random outcome assessor (detection bias): Were animals selected at random for outcome assessment?
Q5: Incomplete outcome data (attrition bias): Were incomplete outcome data adequately addressed?
Q6: Selective Reporting (reporting bias): Are reports of the study free of selective outcome reporting?
Q7: Other Bias: Was the study apparently free of other problems that could result in high risk of bias
Meta-analysis on the mean difference of the efficacy of exenatide as compared to metformin in BMI levels of PCOS patients
Forest plot in figure 2 exhibited the pool effect on the efficacy of exenatide as compared to metformin in reducing the Body Mass Index (BMI) of PCOS patients. The forest plot shows the first author of the article and the year it was published. The statistical analysis portrayed, that the pool effect between the two interventions, exenatide and metformin was (mean difference = 0.51; 95% Confidence Interval (CI)= 0.07, 0.96, I2= 52%). The pool results did not intersect the line of no effect and shows that there was a significant reduction in BMI levels of PCOS patients in exenatide as compared to metformin intervention. The p value is also less than 0.05, which proves the result to be significant (p=0.02).
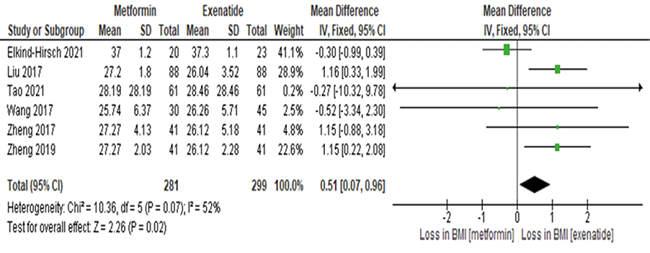
Figure 2. Forest plot of the meta-analysis from six studies showed the overall mean difference of the efficacy of exenatide as compared to metformin in PCOS patients (mean difference = 0.51; 95% Confidence Interval (CI)= 0.07, 0.96, I2= 52%). The pool result did not intersect the line of no effect indicating the result to be significant, where exenatide has a significant reduction in the BMI level of PCOS patients as compared to metformin. The p value is also less than 0.05, which proves the results to be significant (p=0.02).
Meta-analysis on mean difference of the efficacy of exenatide as compared to metformin in testosterone levels of PCOS patients
Forest plot in figure 3 exhibited the pool effect on the efficacy of exenatide as compared to metformin in reducing the Body Mass Index (BMI) of PCOS patients. The forest plot shows the first author of the article and the year it was published. The statistical analysis portrayed, that the pool effect between the two interventions, exenatide and metformin was (mean difference = 0.15; 95% Confidence Interval (CI)= 0.07, 0.22, I 2= 0%). The pool results did not intersect the line of no effect and shows that there was a significant reduction in testosterone levels of PCOS patients in exenatide as compared to metformin intervention. The p value is also less than 0.05, which proves the result to be significant (p=0.0002).

Figure 3. Forest plot of the meta-analysis from six studies showed the overall mean difference of the efficacy of exenatide as compared to metformin in PCOS patients (mean difference was (mean difference = 0.15; 95% Confidence Interval (CI)= 0.07, 0.22, I 2= 0%). The pool result did not intersect the line of no effect indicating the result to be significant, where exenatide has a significant reduction in the testosterone level of PCOS patients as compared to metformin. The p value is also less than 0.05, which proves the results to be significant (p=0.0002).
Meta-analysis on mean difference of the efficacy of exenatide as compared to metformin in LDL levels of PCOS patients
Forest plot in figure 4 exhibited the pool effect on the efficacy of exenatide as compared to metformin in reducing the Low-Density Lipoprotein Cholesterol (LDL-C) of PCOS patients. The forest plot shows the first author of the article and the year it was published. The statistical analysis portrayed, that the pool effect between the two interventions, exenatide and metformin was (mean difference = -0.09; 95% Confidence Interval (CI)= -0.29, 0.12, I 2= 0%). The pool results intersect the line of no effect and shows that there was no significant reduction in LDL cholesterol levels of PCOS patients when compared between exenatide and metformin intervention. The p value is also more than 0.05, which proves the result to be not significant (p=0.40).
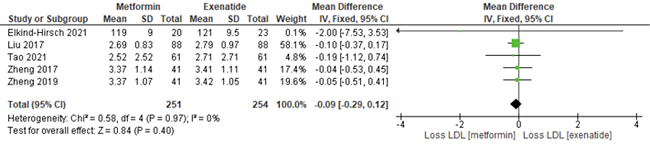
Figure 4. Forest plot of the meta-analysis from six studies showed the overall mean difference of the efficacy of exenatide as compared to metformin in PCOS (mean difference = -0.09; 95% Confidence Interval (CI)= -0.29, 0.12, I 2= 0%). The pool results intersect the line of no effect indicating the result to be not significant, where there was no significant reduction in the LDL-C level of PCOS patients when compared between exenatide and metformin. The p value is also more than 0.05, which proves the results to be significant (p=0.40).
Meta-analysis on mean difference of the efficacy of exenatide as compared to metformin in HDL levels of PCOS patients
Forest plot in figure 5 exhibited the pool effect on the efficacy of exenatide as compared to metformin in reducing the High-Density Lipoprotein Cholesterol (HDL-C) of PCOS patients. The forest plot shows the first author of the article and the year it was published. The statistical analysis portrayed, that the pool effect between the two interventions, exenatide and metformin was (mean difference = 0.06; 95% Confidence Interval (CI)= -0.01, 0.13, I 2= 80%). The pool results intersect the line of no effect and shows that there was no significant reduction in HDL cholesterol levels of PCOS patients when compared between exenatide and metformin intervention. The p value is also more than 0.05, which proves the result to be not significant (p=0.11).
Figure 5. Forest plot of the meta-analysis from six studies showed the overall mean difference of the efficacy of exenatide as compared to metformin in was (mean difference = 0.06; 95% Confidence Interval (CI)= -0.01, 0.13, I 2= 80%). The pool results intersect the line of no effect indicating the result to be not significant, where there was no significant reduction in the HDL-C level of PCOS patients when compared between exenatide and metformin. The p-value is also more than 0.05, which proves the results to be significant (p=0.11).
Discussion
Interpretation of the evidence
There were 6 studies that compared the efficacy between metformin and exenatide. Meta-analysis of the included studies shows that when exenatide was administered, they showed significant improvement in terms of BMI (mean difference = 0.51; 95% Confidence Interval (CI)= 0.07, 0.96, I 2= 52%; p=0.02) and testosterone levels (mean difference = 0.15; 95% Confidence Interval (CI)= 0.07, 0.22, I 2= 0%; p=0.0002), whereas the LDL-C (mean difference = -0.09; 95% Confidence Interval (CI)= -0.29, 0.12, I 2= 0%; p=0.40) and HDL-C (mean difference = 0.06; 95% Confidence Interval (CI)= -0.01, 0.13, I 2= 80%; p=0.11) had no significant improvement.
Efficacy of metformin and exenatide
Exenatide drugs appear to manifest more adverse effects, but the drug is effective in treating and managing the symptoms in PCOS patients by improving the insulin resistance, normalizing the BMI of the patients, reduce food absorption and reducing inflammation than metformin.(1, 70) reported that metformin showed improvements in live births, pregnancy and ovulation rate. According to1 the BMI level of patients under exenatide as compared to metformin showed a better reduction. PCOS patients given exenatide manifested improved menstrual cycle, IR which leads to high chance of getting pregnant. Improvement in insulin resistance was mediated by AMP-activated protein kinase (AMPK) which could be a promising target to cure PCOS.70
There were 2 efficacy endpoints upon the medication. The first efficacy endpoint was that patients reported with prediabetes returned to have normal blood glucose levels. A total of 76 out of 150 patients showed improvements in the blood glucose level upon 12 weeks of medication. The remission rate of prediabetes was 64% under the combination group. The combination of metformin and exenatide drugs showed the highest remission rate to prediabetes and improved menstrual cycle and ovulation rate [10, 70]. Exenatide medication alone had 56% of patients who showed improvements while metformin medication had the lowest improvement rate which was 32%. The secondary endpoint would be improvements in their anthropometric measurements. The reduction in the BMI level was seen in both the drugs. Improvements in the hormonal status such as the testosterone level and their lipid profile such as the HDL-C and LDL-C were also seen under the medication. The testosterone level was also lowered for both the drugs. The level of LDL-C was lowered under metformin and combination drugs but not under exenatide drugs. More LDL-C was lowered in metformin as compared in the combination drugs. The HDL-C level in PCOS patients did not show any improvements from the baseline.10 I have been reported that the fasting and mean blood glucose levels of PCOS patients showed significant improvements under exenatide. (39 The fasting insulin sensitivity tested by the HOMA-IR showed that all drugs showed improvements but PCOS patients under exenatide medication showed the highest response to insulin. The BMI level was also significantly reduced under exenatide medication. The total testosterone levels in all medication groups were reduced while the LDL-C and HDL-C levels did not show any improvements as compared to the baseline.
Adverse effect of the treatment
Metformin and exenatide drugs cause some adverse events upon prescription of medicine. During the study of PCOS patients with metformin, they develop mild to moderate gastrointestinal reactions.70,71 In groups that were prescribed with exenatide, they had nausea which was the common symptoms among the patients. Nausea was from mild to moderate intensity where the symptoms started from 0 to 8 weeks upon prescription. Exenatide medication initially showed more symptoms of nausea in PCOS patients which eventually reduced in numbers as patients were able to tolerate the medication. Vomiting and abdominal distension were also prominently seen in PCOS patients under exenatide. The doses that were gradually increased for both drugs, metformin and exenatide appear to show lesser adverse symptoms.1) PCOS patients under metformin had frequent diarrhea as compared to the combination group. The combination medication of both the drugs exenatide and metformin also has more adverse effects in PCOS patients. The combination group manifest nausea in a more severe level as compared to metformin. Abdominal distension, constipation, vomiting, headache, and fatigue are the common adverse effects of both the metformin and combination drugs.13
Moreover, PCOS patients that were studied with exenatide medication showed pruritus and subcutaneous induration with a diameter of <0.5 cm at the injection site when they were injected subcutaneously. Patients did not drop out from the study although they had itchiness at the site of injection.13
Resistance towards treatment or dropouts from treatment
PCOS patients under exenatide medication had higher dropout rates as the patients could not tolerate gastrointestinal reactions as compared to metformin.1) Patients also dropped out from the treatment due to diarrhea during the course of study.13) PCOS patients prescribed with either exenatide, metformin or combined drugs could not continue in the study being due to their failure in following the 80% of dosage prescribed and where they reduced the dosage on their own without getting consent. Patients also fail to take the medicines on time as being prescribed, making them unable to continue in the study too. Patients also had to drop out from the study as some had adverse reactions where 2 patients from exenatide and metformin had nausea and vomiting while 1 patient with headache under metformin treatment.10 Apart from that,39 reported that around 4 PCOS patients became pregnant while the study was conducted making them unable to continue the study.
Similarities and Differences in the efficacy between metformin and exenatide
PCOS patients are not only characterized by hyperandrogenism and infertility but are also associated with metabolic abnormalities like prediabetes which has a higher possibility to progress to type 2 diabetes. Insulin resistance is one the main pathophysiology in of prediabetic in PCOS patients.10 Obesity mainly abdominal obesity in PCOS women is highly associated with metabolic disorders.72 In a present study, both exenatide and metformin drug significantly reduces the levels of HbA1c in obese or overweight patients, but exenatide was observed to work more effectively as compared to metformin.73) Exenatide and metformin drug was also proved to reduce weight in PCOS patients, but the combination therapy of the drug proved to be more effective in reducing the weight of PCOS patients.72 Exenatide drug is a Glucagon-like peptide-1 (GLP-1) receptor agonist. GLP-1 receptor agonist works the best for type 2 diabetes patients and was also proved to reduce the weight in obese or overweight patients more efficiently than metformin.73) Also, metformin works by suppressing hepatic gluconeogenesis and improve the levels of fasting blood glucose whereas exenatide reduces plasma glucose level by inducing the β - cell insulin secretion. Therefore, if PCOS women who are at their reproductive age, can manage the weight by either intervention or lifestyle modification then the prediabetic state of PCOS can be managed and prevent the progressing to type 2 diabetes. Also, weight loss in PCOS patients can aid in reducing hyperinsulinemia and hyperandrogenism.72
Insulin resistance and hyperandrogenisms in PCOS
A recent study has stated that increased phosphorylation of insulin-stimulated association of IRS-1 at the main regulatory site of the Ser312 was observed in PCOS patients which leads to the disturbance in insulin signaling.74) In PCOS patients, ovaries produce 60% of androgen and adrenal glands produce the remaining 40% of androgen and this leads to hyperandrogenemia in PCOS patients. The GnRH agonists were found to disturb the androgen synthesis in the ovaries of PCOS women which leads to higher level of androgen hormone in their blood. Also, it was found that dexamethasone disturbs the androgen synthesis of the adrenal glands of PCOS women which leads to higher level of androgen hormone in their blood. Lower level of sex-hormone binding globulin (SHBG) leads to increased level of testosterone in the blood because the hormone is unable to bind to the receptor and remains in the blood. In addition, a recent study found that a drug diazoxide, reduces insulin production without altering the insulin sensitivity. This also leads to increased SHBG levels in PCOS women. This scenario explains clearly why obese PCOS women tend to have low levels of SHBG.74
Obesity and PCOS
Obesity is closely related with the manifestation of symptoms by PCOS women, especially insulin resistance. A study states that, PCOS women were highly affected by obesity, inherited insulin resistance and extrinsic insulin resistance due to unhealthy lifestyle. PCOS women, mostly have higher abdominal obesity which is correlated to insulin resistance. Both obesity and insulin resistance increase the symptoms and manifestation of the disease, PCOS.75 A study by 76 states that obesity aggravates glucose intolerance rate, therefore obese PCOS women tend to manifest high rate of glucose intolerance. Obese women are mostly observed to have menstrual irregularities and anovulatory infertility. PCOS women with BMI more than 24 kg/m2 are mostly being diagnosed with anovulatory infertility. Moreover, PCOS women normally has increased androgen levels and obesity, especially central obesity worsens the symptoms due to low levels of SHBG levels due to hyperinsulinemia.76) Overweight or obese PCOS women of all ages have low levels of Sex-Hormone Binding Globulin (SHBG) than PCOS women who has normal BMI. Abdominal obesity is highly related to low levels of SHBG. Obese or overweight PCOS women have reduced levels of SHBG and increased levels of total testosterone, fasting insulin and glucose levels and HOMA-IR which proves that the BMI of the PCOS women have a significant influence in insulin resistance and hyperandrogenism. A recent study confirms that increased insulin level in the body reduces the SHBG level by disturbing the hepatic production of SHBG which eventually increased the level of free testosterone androgen.77-78 Increased level of free testosterone also leads to insulin resistance (IR). Therefore, PCOS women should focus in managing the weight and to normalize their BMI levels by engaging themselves in physical activity and practice healthy diet. By doing this PCOS women can improve hyperandrogenism, ovulation, menstruation and increase SHBG levels in the blood.77)
Risk of Bias (RoB) Assessment
This meta-analysis focuses on human studies and in vivo studies. A total of 57 articles were retrieved and mostly the studies involved were human studies. The risk of bias for human study was done using the Cochrane tool.16) Three studies were observed to be high risk bias with low quality of studies. A study21 showed high risk in random sequence generation (selection bias) because there was no randomization for the placebo, blinding of personnel/care providers (performance bias) was also high risk because there was no blinding of participants as they were aware of the intervention given to them and other bias was also high risk because patient aging process disturbed the outcome of the study. Unclear bias was reported for allocation concealment (selection bias), blinding of outcome assessor (detection bias) and incomplete outcome data (attrition bias) as there were insufficient reporting of data and information. A study 24 showed high risk of bias in random sequence generation (selection bias) because no randomization of study group was done, allocation concealment (selection bias) was high risk as the physicians were aware of the allocation of intervention, blinding of personnel/ care providers (performance bias) was also high as the participants were not blinded, blinding of outcome assessor (detection bias) also had high risk as outcome assessors were not blinded and other bias was also high risk as in retrospective cohort study is highly prone to misclassification bias. Moreover, a study30 showed high risk of bias in random sequence generation (selection bias) because no randomization of study group was done as it was an open label study, allocation concealment (selection bias) was high risk as the physicians were aware of the allocation of intervention as it was an open label study, blinding of personnel/ care providers (performance bias) was also high as the participants were not blinded since it is an open label study, blinding of outcome assessor (detection bias) also had high risk as outcome assessors were not blinded due to the open label study. The RoB analysis for in vivo study was done using the Systematic Review Centre for Laboratory Animal Experimentation (SYRCLE) tool.17) All 4 studies reported to have high risk of bias where it had low quality of studies. The study59,62,64,66 showed high risk of bias in random sequence generation (selection bias) because no randomization of animal group was done, allocation concealment (selection bias) was high risk as the investigators can foresee the animal groups allocated for intervention, blinding of personnel/ care providers (performance bias) was also high as the animals were not housed randomly, blinding of outcome assessor (detection bias) was also high as the animals were not selected at random for outcome assessment.
Limitations of the study
The meta-analysis was subjected to a few limitations. The studies that were included had a smaller sample size. Smaller sample size can lead to false result where the real efficacy of the drugs could not be properly concluded.79) To be precise small sample size can lead to false positive result. This could lead to the participation of the study group to be not benefited and does not provide benefit to future patients and for the improvement and development of science.80 Small sample size of study can lead to defect in the result interpretation especially in terms of p-value and 95% confidence interval value.81) The next limitation is that the study is single centered study. The issue in single-centered study design is the limited external validity. The efficacy of the intervention done in a single centered study design could not generalize the efficacy of the intervention to an entire population.82) Moreover, the studies included were also observed to have a shorter duration of intervention. Shorter duration of intervention can restrict the researchers from observing and comparing the sustainability of the drugs.10 Also, articles that were only published in English were included due to the language barrier.
Conclusion and Future recommendation
This meta-analysis summarizes that the drug, exenatide has a significant reduction on the level of BMI and testosterone when compared to the drug, metformin (mean difference = 0.51; 95% Confidence Interval (CI)= 0.07, 0.96, I 2= 52%; p=0.02) and (mean difference = 0.15; 95% Confidence Interval (CI)= 0.07, 0.22, I 2= 0%; p=0.0002). Also, there were no significant effect on the level of low-density lipoprotein cholesterol (LDL-C) and high-density lipoprotein cholesterol (HDL-C) when compared between metformin and exenatide (mean difference = -0.09; 95% Confidence Interval (CI)= -0.29, 0.12, I 2= 0%; p=0.40) and (mean difference = 0.06; 95% Confidence Interval (CI)= -0.01, 0.13, I 2= 80%; p=0.11). All the findings suggested that insulin resistance and obesity worsen the symptoms in polycystic ovarian syndrome (PCOS) women. Reducing weight in PCOS women can improve insulin resistance.39) Therefore, an effective intervention like, exenatide and proper weight management and healthy lifestyle should be practiced by PCOS women in order to prevent further complications.75,77
One of the recommendations for future research would be that longer duration of intervention should be implemented while observing the response from PCOS women.10) Longer duration of intervention can yield, an increased number of precise outcomes.83) It is also suggested to conduct the research with a larger number of study group diagnosed with PCOS, as larger sample size can prevent false positive results and prevent misinterpretation of the result obtained.(80, 81) Also, it is best if the research could focus into multi-ethnic or multi-centered groups in order to confirm the findings of the research as it can help to prove to be effective to all PCOS women populations.10) Different people respond differently to the same drug, therefore focusing into multi-ethnic or multi-centered groups can provide a proper result, as we can observe, and study different reactivity of different ethnic groups and future research can focus into more effective dosage of the drug or even better intervention to cure PCOS can be identified.