Meu SciELO
Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
REC: Interventional Cardiology
versão On-line ISSN 2604-7276versão impressa ISSN 2604-7306
REC Interv Cardiol ES vol.5 no.1 Madrid Jan./Mar. 2023 Epub 18-Mar-2024
https://dx.doi.org/10.24875/recic.m22000342
SPECIAL ARTICLE
Plaque modification techniques to treat calcified coronary lesions. Position paper from the ACI-SEC
aServicio de Cardiología, Hospital Universitario La Paz, Madrid, España
bServicio de Cardiología, Hospital Juan Ramón Jiménez, Huelva, España
cInstituto Cardiovascular, Hospital Clínico San Carlos, Madrid, España
dServicio de Cardiología, Hospital Universitario de Salamanca, Instituto de Investigación Biomédica de Salamanca (IBSAL), Salamanca, España
eServicio de Cardiología, Hospital Universitario Lucus Augusti, Lugo, España
fServicio de Cardiología, Hospital Universitario Reina Sofía, Universidad de Córdoba, Instituto Maimónides de Investigación Biomédica de Córdoba (IMIBIC), Córdoba, España
gServicio de Cardiología, Hospital La Luz, Madrid, España
hInstitut del Cor, Hospital Universitari Germans Trias i Pujol, Badalona, Barcelona, España
iServicio de Cardiología, Hospital del Mar, Barcelona, España
jInstitut Clínic Cardiovascular, Hospital Clínic, Institut d'Investigacions Biomèdiques August Pi I Sunyer (IDIBAPS), Barcelona, España
kServicio de Cardiología, Hospital Clínico de Santiago de Compostela, Santiago de Compostela, A Coruña, España
Abbreviations
>CB: |
cutting balloon. |
ELCA: |
excimer laser coronary angioplasty. |
ICL: |
intracoronary lithotripsy. |
OA: |
orbital atherectomy. |
RA: |
rotational atherectomy. |
SB: |
scoring balloon. |
IMPLICATIONS OF CALCIFICATION IN PERCUTANEOUS CORONARY INTERVENTIONS
Vascular calcification is a process closely associated with atherosclerosis. It can occur in the media (in peripheral arteries mainly) or intima layers (in coronary arteries). In the context of coronary atherosclerosis it debuts in intermediate or advanced stages in plaque evolution due to conversion of smooth muscle cells into osteoblastic phenotypes and infiltration of atheromatous plaque due to macrophages that clear out apoptotic smooth muscle cells and contain calcified vesicles.1 Atheromatous plaque calcification can take different shapes that probably correspond to different stages of the same disease like microcalcifications (< 15 μm), punctiform calcifications (circumferential arc < 90º), leaves or thin calcium layers (circumferential arc > 90º or > 3 mm in length), and calcium nodules.1
The main risk factors associated with coronary artery calcification are age, Caucasian race, diabetes mellitus, and chronic kidney disease.1
The prevalence of coronary artery calcification if variable based on the population studied and the diagnostic method used.2 The traditional angiographic definition of moderate calcification described radiopacities seen during cardiac motion while severe calcification is described as radiopacities seen without cardiac motion, usually on both sides of the arterial lumen. The prevalence of moderate or severe calcification is between 18% and 60%.3,4
Calcification complicates percutaneous coronary interventions (PCI) for various reasons: a) resistance to the advance of different devices especially in the presence of tortuosity (eventually, non-crossable lesions); b) reduced plaque compliance that will eventually require higher pressure in dilatation balloons or plaque modification devices (non-dilatable lesions); and c) difficulties advancing the stent and expanding it.5 Other issues would be malapposition and polymer damage that can lead to a non-homogeneous release of antiproliferative drugs. Everything combined makes calcification one of the major determinants of the SYNTAX score,6 and associated with worse PCI outcomes and higher rates of adverse events at follow-up including mortality in patients with extremely calcified coronary artery lesions.7 In addition, it increases the rate of procedural complications associated with calcification per se and with the tools necessary for treatment: coronary artery dissection, loss of side branches, PCI material entrapment, stent distortion or even stent loss, and the dreaded coronary artery perforation that is particularly severe since it is very difficult to advance any kind of sealing materials.8
To stop these issues and their prognostic implications from happening numerous plaque modification devices have been developed. The appropriate use of these devices is essential to perform safe and effective PCIs on calcified coronary artery lesions.
This position paper has been promoted by the Interventional Cardiology Association of the Spanish Society of Cardiology (ACI-SEC) with contributions from different expert professionals in this setting. It describes the plaque modification techniques currently available in our field and proposes an algorithm for the management of calcified coronary artery lesions.
INTRACORONARY IMAGING MODALITIES FOR CALCIFIED LESION ASSESSMENT
Intracoronary imaging modalities play a key role in the assessment of calcified coronary artery lesions. The use of optical coherence tomography (OCT) or intravascular ultrasound (IVUS) can be very useful to improve the detection and assesment of coronary artery calcium, select the plaque modification technique, and optimize results especially in association with stent expansion.
Calcification detection and assessment
Angiography is a limited sensitivity tool to detect coronary artery calcium. Unlike angiography both the IVUS and the OCT have higher sensitivity and specificity to assess the characteristics and degree of calcification, which are basic aspects to determine the therapeutic options.2,9 Table 1 shows the differences of these 2 intracoronary imaging modalities regarding calcium detection. The main difference between the 2 is that, since calcium creates posterior acoustic shadowing on the IVUS, calcium thickness cannot be properly assessed. As alternative marker, the presence of reverberations on the IVUS has been associated with the presence of thinner calcium layers (< 0.5 mm). On the OCT, parietal calcium does not create posterior acoustic shadowing and, therefore, its thickness can be assessed accurately. Nodular calcium, however, creates a shadow in both the IVUS and the OCT (figure 1).
Table 1. Intracoronary imaging modalities for calcified coronary artery lesion calcification
| Imaging modality | Sensitivity | Specificity | Calcium pattern | Calcium arc | Calcium length | Calcium thickness | Disadvantages |
|---|---|---|---|---|---|---|---|
| OCT | ++++ | ++++ | Parietal calcium: low reflectivity structure with demarcated borders and without posterior shadowing (figure 1A) Calcium nodule: Protruding structure into the lumen with posterior shadowing (figure 1C) | Allows quantification | Allows quantification | Can be measured | Requires clearing the blood from the vessel lumen for image acquisition. This can increase the contrast volume compared to IVUS Does not acquire proper images of ostial lesions |
|
| |||||||
| IVUS | +++++ | ++++ | Parietal calcium: hyperechogenic structure with posterior shadowing (figure 1B) Calcium nodule: Structure protruding into the lumen with posterior shadowing (figure 1D) | Allows quantification | Allows quantification | Cannot be measured due to posterior shadowing Reverberations are a marker of thin calcium (< 0.5 mm) | Posterior shadowing complicates calcium thickness assessment In the 20 MHz IVUS the limited resolution and near-field clutter artifact can complicate the definition of calcium depth with respect to lumen in severe lesions |
IVUS, intravascular ultrasound; OCT, optical coherence tomography.
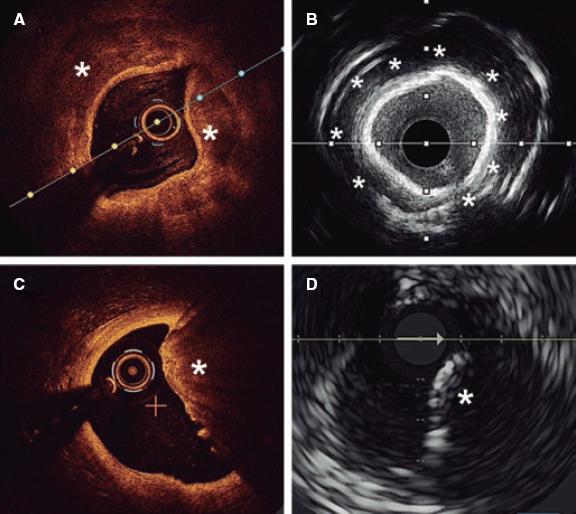
Figure 1. Coronary artery calcium assessment with IVUS and OCT. A: Parietal calcium on the OCT, low reflectivity structure with demarcated borders (asterisk). B: Parietal calcium on the IVUS, hyperechogenic structure with posterior shadowing. C: Calcium nodule on the OCT, structure protruding into the lumen with posterior shadowing. D: Calcium nodule on the IVUS, structure protruding into the lumen with posterior shadowing.
Different scoring systems have been developed for both intracoronary imaging modalities (table 2) including the characteristics of calcification that have been associated with stent underexpansion. The first OCT suitable scale ever developed includes 3 different parameters: calcium arc > 180º (score = 2), length > 5 mm (score = 1), and thickness > 0.5 mm (score = 1). Lesions with scores > 2 have a higher risk of stent underexpansion if proper plaque preparation is lacking.5 A similar scale has been developed for IVUS using 4 different criteria: calcium arc > 270º with > 5 mm in length (score = 1), calcium arc > 360º (score = 1), presence of calcified nodule (score = 1) and adjacent vessel < 3.5 mm (score = 1). Scores ≥ 2 are indicative of the need for plaque modification prior to stenting.10
Table 2. Intracoronary calcium scores based on optical coherence tomography and intravascular ultrasound
| OCT | IVUS | |||
|---|---|---|---|---|
|
| ||||
| Scores | Scores | |||
| Máximo arco de calcio | ≤ 180° | 0 | ≤ 270º | 0 |
|
| ||||
| > 180° (> 50%* of arc circumference) | 2 | 270º and > 5 mm in length | 1 | |
|
| ||||
| 360º | 1 | |||
|
| ||||
| Máximo grosor de calcio | ≤ 0.5 mm | 0 | ||
|
| ||||
| > 0.5* mm | 1 | |||
|
| ||||
| Longitud de calcio | ≤ 5 mm | 0 | ||
|
| ||||
| > 5* mm | 1 | |||
|
| ||||
| Type of calcium | Non-nodular | 0 | ||
|
| ||||
| Nodule | 1 | |||
|
| ||||
| Vessel diameter | ≥ 3.5 mm | 0 | ||
|
| ||||
| < 3.5 mm | 1 | |||
IVUS, intravascular ultrasound; OCT, optical coherence tomography. Scores ≥ 2 on the IVUS and > 2 on the OCT are suggestive when using calcium modification techniques due to the risk of stent underexpansion.
*Rule of 5: Lesions where calcium occupies > 50% of the arc circumference extend longitudinally > 5 mm and have > 0.5 mm of thickness require advanced calcium modification techniques.
Selection of plaque modification techniques under intracoronary imaging modality guidance
The characteristics of calcium as seen on the intracoronary imaging modalities can contribute to the selection of the most adequate plaque modification technique. There is in depth information on this aspect in the last section of the document but, overall, lesions where calcium does not have underexpansion risk criteria can be treated with high-pressure or modified balloons (scoring, cutting). However, if these criteria exist it will be necessary to use more advanced plaque modification techniques. Added to these criteria, we should also mention calcium depth since some imaging modalities only act on the superficial not deep layer of the plaque.
Optimization of stenting under intracoronary imaging modality guidance
Both the IVUS and the OCT allow us to determine whether proper stent expansion has been achieved. This is especially relevant in calcified coronary artery lesions that happen to be the ones that are most associated with stent underexpansion, the parameter most strongly associated with stent failure.11 Proper apposition and lack of dissection or significant border hematoma, as well as proper lesion coverage are other optimization parameters under intracoronary imaging modality guidance that should also be assessed after stenting.12
BALLOON-FREE TECHNIQUES
Rotational atherectomy
The rotational atherectomy (RA) technique uses a metal olive-shaped burr covered with diamond crystals in its distal third that rotates at high speed and performs a differential cut when advancing through the vessel (figure 2A) while pulverizing calcified tissue and preserving the adjacent elastic tissue.13
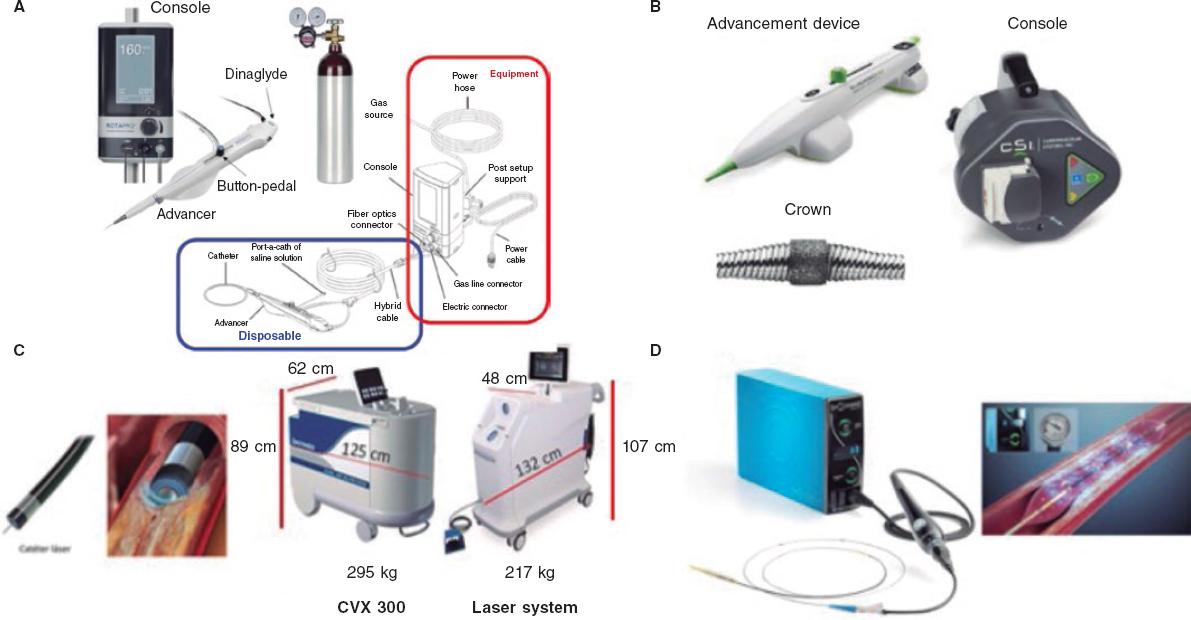
Figure 2. Plaque modification devices. A: Rotational atherectomy device. B: Orbital atherectomy device. C: Coronary laser device with the 2 existing console models. D: Intracoronary lithotripsy device. Modified with permission from Cubero-Gallego et al.13
It appeared over 30 years ago to facilitate the management of coronary artery lesions by reducing plaque burden. Early enthusiasm turned into an elevated use of RA in different settings without the proper scientific back-up. This triggered suboptimal results14 that reduced its use to highly selected cases only. Through all these years, RA has evolved with technological improvements, and also of the technique itself, as well as the selection of patients.
Currently, the ROTAPRO system (Boston Scientific, United States) is available. It has made the technique easier because it has replaed the pedal of the early version for a button placed on top of the olive-shaped burr advancer. There is another button on the side of the advancer to change to the Dynaglide mode (rotation at low revolutions is advised to introduce and remove the burr). Console is smaller and comes with a digital screen. Size of the burrs is between 1.25 mm and 2.5 mm, and they are compatible with 6-Fr-to-8-Fr catheters based on the size of the olive-shaped burr that advances on a 0.009 in specific guidewire (0.014 in the radiopaque side) of which 2 different versions exist (RotaWire Floppy and RotaWire Extra-Support) that should be used depending on the characteristics of the plaque and support needed.13
The main indication is to treat extremely calcified non-crossable or non-dilatable coronary artery lesions with balloon. Probably, the optimal scenario is a concentric calcified lesion with a smaller luminal area compared to the olive-shaped burr. Eccentric angulated lesions are less favorable since they are associated with a higher risk of complications.13,15 It can be used as a primary or a bailout strategy after balloon failure. The primary strategy has been associated with shorter procedures, less radiation and contrast, and probably lower cost regarding the material used.15
The target of RA has also changed from the old idea of removing as much plaque as possible (debulking) to the modern approach of modifying plaque to facilitate the PCI. Technical recommendations to perform RA have evolved too. Current recommendations are shown on table 3.16
Table 3. Recommendations for a safe use of rotational atherectomy
| Arterial access | It depends on the maximum size of the olive-shaped burr. Currently, the most widely used is radial access because it allows the use of burrs of up to 1.75 mm (when using a 6-Fr catheter) or 2.15 mm (when using a 7-Fr catheter) |
|
| |
| Guide catheter | High-support catheters with a simple curve are advised |
|
| |
| Guidewire | Direct guidewire placement is often feasible, although a conventional guidewire can be used, and exchange performed using a microcatheter or a coaxial balloon Based on the lesion characteristics, the RotaWire Floppy or Extrasupport can be used |
|
| |
| Size of olive-shaped burr | The use of small burrs is advised to keep the burr/artery ratio ≤ 0.5. The size of the most widely used burr is 1.5 mm. In some cases, the gradual increase of the size of the burr is advised |
|
| |
| Rotablation speed | Selection of rotablation speeds < 180 000 rpm ideally between 135 000 rpm and 150 000 rpm is advised. High speeds should be spared for cases where the burr cannot cross despite using the optimal technique available. Special attention should be paid to avoid drops > 5000 rpm during rotablation |
|
| |
| Ablation time | Shorter ablation times (ideally ≤ 15 seconds) reduce the risk of complications (atrioventricular block, flow slowing down) |
|
| |
| Ablation motion | Gradual, and continuous pecking motion |
|
| |
| Cleansing serum | Heparinized saline solution should be used with vasodilators/spasmolytics (verapamil, nitrates) |
|
| |
| Pacemaker | The use of olive-shaped burrs of smaller diameter, lower speeds, and the position of the burr with the Dynaglide mode have proven to reduce the number of transient atrioventricular blocks during rotablation substantially In selected cases, above all, in dominant right coronary or left circumflex arteries the preventive use of IV atropine or transient pacemaker implantation can be considered |
The most common complication is slow/no-flow although its rate has dropped down to 2.6%.17 It is due to debris embolization towards microcirculation and there is higher risk in long lesions where multiple and prolonged passes with large olive-shaped burrs are performed without proper pauses among them and in the presence of a poor distal vessel. The management of dominant right coronary or left circumflex coronary arteries can be associated with transient conduction disorders. Severe complications like burr entrapment, perforation, and coronary dissection are rare.13 Factors predisposing burr entrapment are lesion severity, steep angulations, and the use of very small burrs. Tortuosity and the lack of guide catheter coaxiality in the management of ostial lesions can trigger dissections and coronary perforations.
Although RA has demonstrated that it facilitates PCI with higher rates of success compared to balloon angioplasty, No clinical benefit has been yet confirmed.18-21
To analyze its results we should mention that RA has been used in patients of higher clinical risk with more complex coronary artery lesions.22 Another aspect we should take into consideration is the high percentage of cases where this technique was used as a bailout strategy (12% to 50%)20,21,23 meaning that without RA these cases would not have been performed or had had worse results. Although ongoing trials are studying the advantages of elective or bailout RA, proper patient and lesion assessment should lean towards increasing its elective or earlier use with a potential beneficial impact on clinical outcomes.24
In conclusion, when performed under the current recommendations RA is a safe and effective procedure. It should become part of our therapeutic arsenal in our cath labs with trained personnel for proper use.
Orbital atherectomy
The Diamonback-360 (OAS) device (Cardiovascular Systems, United States) is a diamond-coated bidirectional orbital crown that uses a combination of centrifugal force (by creating elliptical orbits) and friction to the surface to modify the calcified plaque and increase compliance (figure 2B). Also, with the pulsatile impact of the crown after speeding up, microfractures can occur that eventually modify deep calcium layers (figure 2B and figure 3). That is why a single 1.25 mm crown can treat vessels from 2.5 mm up to 4 mm.
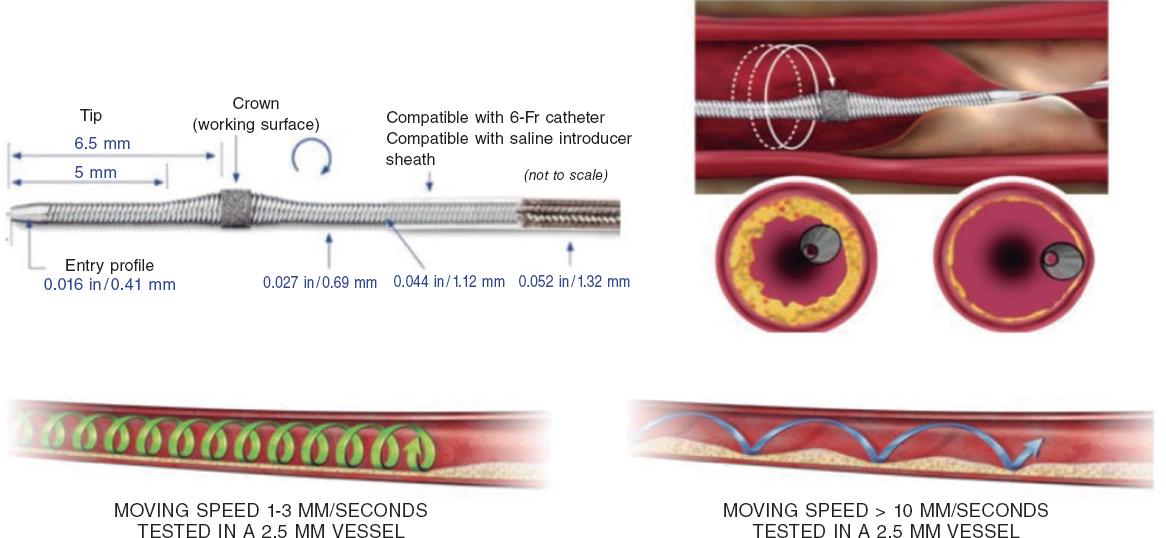
Figure 3. Characteristics of orbital atherectomy catheter and its effects. (Modified with permission from Cardiovascular Systems.25)
Compared to the remaining plaque modification techniques, this orbital atherectomy (OA) has arrived late to our country and our experience is still scarce.
Its main indication is to treat no-dilatable calcified coronary artery lesions.26
Preparation is very similar to RA, but here a specific guidewire is needed, the Viper-Wire. Crown advances with the Glide-Assist system (rotation at low revolutions) until coming close to the lesion. Another distinctive feature of this device is its antegrade and retrograde ablation functionalities. Unlike RA, the speed at which the device moves forward needs to be very slow (between 1 mm and 3 mm per second) to guarantee good procedural results and reduce complications.17,26 The mechanism of action of OA consists of the crown elliptical rotation that achieves a gradual increase of orbital diameter as rotation speed increases from 80 000 rpm up to 120 000 rpm. Cycles ≤ 30 seconds are advised (it comes with a sound warning signal to end the cycle) with pauses between 20 and 30 seconds among them that can duplicate in cases of poor hemodynamic tolerance.26 The continuous infusion of a lubricant solution is necessary to minimize thermal lesions during OA. Also, 18 mL/min are administered to cool the device down and get rid of debris, thus reducing the chances of ischemia and distal embolization.13,26,27
Complications are similar to those of RA. However, the possibility of retrograde application reduces the chances of crown entrapment and the risk of dissection or perforation in angulated or ostial lesions. The rate of perforation is between 0.7% and 2%.28,29 Theoretically speaking, the debris created by OA is smaller compared to the debris created by RA. This added to the fact that the crown does not stop coronary flow during atherectomy reduces the risk of slow/no-reflow and endothelial thermal lesion.27 However, transient conduction disorders are not rare when dominant right coronary or left circumflex arteries are treated.
Current evidence available is based on the ORBIT I30 and ORBIT II28 clinical trials where OA obtained good results regarding procedural success (94% and 89%, respectively) with higher rates of major adverse cardiovascular events (MACE), and target lesion failure of 23.5% and 7.8%, respectively, at 3 years.31 Afterwards, the COAST trial29 was conducted where the new MicroCrown system was used. A total of 100 patients were included with rates of procedural success and MACE of 85% and 22.2%, respectively, at 1-year follow-up. We are waiting to see the results from the ECLIPSE trial that will randomize a total of 2000 patients with severe calcifications to receive OA or balloon predilatation prior to by drug-eluting stent implantation.
In conclusion, OA is another calcium plaque modification technique with potential technical advantages like having only 1 size of crown compatible with 6-Fr to treat all lesions and with pull-back capabilities. Although there are insufficient data from comparative studies, choosing this technique will depend on the profile of the patient and the lesion to be treated, the intracoronary being an essential aspect.
Excimer laser
Excimer laser coronary angioplasty (ELCA) is based on a xenon chloride laser that generates short ultraviolet pulses of 308 mm that only penetrate 50 µm in depth, which makes it safer compared to old continuous-wave-near-infrared lasers. It modifies the plaque through a triple mechanism: photochemical (by breaking molecular binds), photothermal (through tissue vaporization), and photokinetic (through the expansion and collapse of the bubble of the catheter tip as it moves forward). Fragments released are < 10 μm, which minimizes microvascular damage after being absorbed by the reticuloendothelial system.
The system currently used is the CVX-300 Laser System (Philips) although there is already a new generation one, the LAS-100 Laser System (Philips) that will be replacing it shortly (figure 2C). There are different sizes of catheters available (0.9 mm, 1.4 mm, 1.7 mm, and 2.0 mm) (table 4). The selection of the catheter depends on the type of lesion and size of the vessel (catheter to vessel diameter ratio, 0.5-0.6) being the 0.9 mm catheter the most widely used for its lower profile and because it can reach higher fluency (80 mJ/mm2), pulse repetition rate (80 Hz), and longer application durations (10 seconds with 5-second rests), which increases the chances of success in fibrous calcific plaques.32,33
Table 4. Characteristics of Excimer laser coronary angioplasty catheters
| 0,9 mm-X 80 | 1,4 mm | 1,7 mm | 2 mm | |
|---|---|---|---|---|
| Compatible guide catheter | 6-Fr | 6-Fr/7-Fr | 7-Fr | 8-Fr |
|
| ||||
| Minimum vessel diameter (mm) | 2 | 2.2 | 2.5 | 3 |
|
| ||||
| Energy (mJ/mm2) | 30-80 | 30-60 | 30-60 | 30-60 |
|
| ||||
| Frequency (Hz) | 25-80 | 25-40 | 25-40 | 25-40 |
|
| ||||
| Application/pause time (seconds) | 10/5 | 5/10 | 5/10 | 5/10 |
Before being used, it is necessary to calibrate the console and then the catheter. In both cases, health professionals and patients should use protective glasses to prevent eye damage. Afterwards, a 0.014 in intracoronary guidewire is inserted until it reaches the lesion. There is a monorail system to facilitate moving forward. Energy is released through the catheter distal border as it slowly advances (0.5 mm/second) to modify the plaque. Catheter withdrawing can also be applied. It is important to optimize support to ensure that the catheter advances. There is no limit in the number of pulses that can be administered since the more it is used, the stronger the effect. However, there is also a higher risk of complications involved. Some authors suggest a maximum of 12 applications.33 The state of the vessel should be assessed after each application. Regarding the selection of parameters, traditionally it started at 45 mJ/mm2, and 25 Hz. However, more and more operators prefer higher energies and initial frequencies especially to treat resistant or calcified lesions.33
Before and during the applications, the blood vessel should be washed, and contrast administered through the infusion of a physiological saline solution (1 mL/s to 3 mL/s). In resistant lesions with severe calcification or underexpanded stents, more energy may be needed. This can be reached by not washing the blood with the physiological saline solution or even administering contrast during applications (the so-called explosion technique). This technique reaches maximum power although it increases the chances of complications. Some authors33 recommend it as the first option to treat non-thrombotic lesions, although it seems reasonable to spare it for ELCA-resistant lesions with saline infusion.
Traditionally, the indications for ELCA have been categorized into 2 different groups: thrombotic (not discussed in this document) and calcified lesions (non-thrombotic like in-stent restenosis, chronic total coronary occlusions, calcified lesions, etc.). The latter can be categorized into non-crossable or non-dilatable lesions:
Non-crossable lesions
The laser main advantage is that it is compatible with all intracoronary guidewires. Therefore, non-crossable lesions with balloon/microcatheter are its main indication.17 In a multicenter registry of non-crossable lesions, the rate of procedural success was 87.3% with 0.8% of dissections showing an impaired flow and no perforations.34 Severe calcification has been associated with a higher probability of technique failure34 since ablation is primarily performed in the tissues between calcium.35 However, the use of ELCA with contrast can increase its chances of success in these lesions.33
Non-dilatable lesions
Although the success of ELCA in non-dilatable lesions is high,36 it has never been the first-line therapy. Among these lesions, an interesting scenario is in-stent lesions (restenosis or underexpansion). In acute underexpansion, ELCA could be the treatment of choice. It allows the modification of resistant tissue located behind the stent without changing its architecture. Its use with contrast can be safer thanks to the stent protective effect. Isolated cases and small case series with success rates > 95% and few complications have been published.37
It is a safe technique when the recommendations given are observed. Coronary artery dissection is the most common complication (5), although it is rarely flow-limiting (1%). The rate of coronary artery perforation is < 1%,38 and distal embolizations and ventricular arrhythmias are exceptional.39
In conclusion, ELCA is especially useful to treat non-crossable lesions thanks to its compatibility with all kinds of angioplasty guidewires. It has also proven effective to treat non-dilatable lesions including in-stent lesions. However, there is still scarce information on its efficacy in calcified coronary artery lesions.
BALLOON-BASED TECHNIQUES
Cutting and scoring balloons
Cutting balloons (CB) are plaque modification devices that appeared as an alternative to old coronary angioplasty balloons.40 Their objective is to achieve controlled ruptures of the plqeu (through incisions in fibrocalcific tissue) (figure 4), thus facilitating balloon expansion, minimizing damage to the intima layer, and reducing stenosis.18,41

Figure 4. Rotacutting technique. Rotational atherectomy (RA) effect and cutting balloon (Rotacutting) with greater plaque modification and minimum lumen area.
There are 2 different types: CB and scoring balloon (BS). Their use has been described in different settings like in-stent restenosis, aorto-ostial lesions, bifurcations, and small vessels associated with the use of drug-eluting stents.42
The main limitations of CBs are their worst navigability and crossing profile compared to conventional balloons. However, over the past few years, both aspects have improved. SBs are associated with better navigability compared to old CBs.
The most dreaded complication is the rupture of coronary artery, although it has significantly increase following its use.
The main difference among the different devices lays in their different external atherotomy elements as described herein (figure 5).
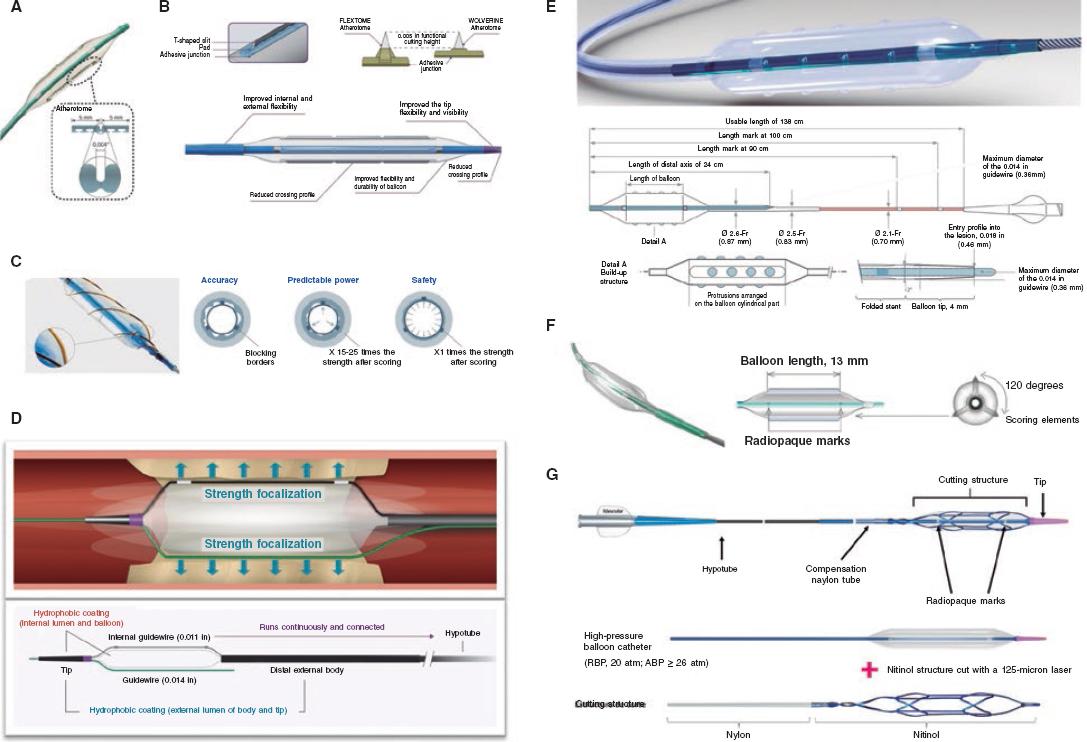
Figure 5. Characteristics of modified balloons. A: Cutting balloon (Boston Scientific, United States). B: WOLVERINE (Boston Scientific, United States). C: AngioSculpt (Spectranetics, United States). D: Scoreflex (OrbusNeich, Hong Kong). E: Grip (Acrostak, Switzerland). F: NSE-Alpha (B.Braun, Germany). G: Naviscore (iVascular, Spain).
Cutting Balloon Flextome
Cutting Balloon Flextome (Boston Scientific, United States) consists of a noncompliant (NC) balloon with 3 microrazors longitudinally mounted on the surface. Its superiority over conventional balloons in A/B lesions has not been confirmed yet, which is why, so far, its use is limited to complex17 and calcified lesions only.43
WOLVERINE
Wolverine (Boston Scientific, United States) is an evolution of the former one with a better crossing profile, greater flexibility, and a more visible tip.
AngioSculpt
AngioSculpt (Spectranetics, United States) is a semicompliant balloon with low crossing profile surrounded by 3 nitinol filaments arranged in a helical cage to secure balloon anchorage. There is a lower risk of dissection or perforation associated with this device.17 It provides more flexibility and better navigability compared to old CBs,44 as well as good results compared to dilatation with semicompliant balloons.45
Scoreflex
Scoreflex (OrbusNeich, Hong Kong) is a NC consisting of a NC balloon with a nitinol dual-wire system to facilitate the controlled modification of the plaque at low pressures. It has a low profile and a combination of hydrophilic and hydrophobic coating that minimizes friction during lesion crossing.
Grip
Grip (Acrostak, Switzerland) is a high-pressure balloon with 4 rows of 3 or 4 knobs in each row. It allows dilatations of up to 22 atm. It comes with a cone-shaped tip in 2 different versions: Grip with a short 2 mm tip, and Grip TT with a long 4 mm tip for greater navigability in tortuous anatomies. It comes with a hydrolubricated coating on its tip and the catheter (not on the balloon), which facilitates both its anchorage to the lesion and navigability across lesions.
NSE Alpha
NSE Alpha (B. Braun, Germany) is a SB with 3 nylon scoring elements and 1 triangular cutting section connected in both borders of the balloon and arranged in a 120º disposition. We should mention its flexibility and navigability with good results in de novo lesions and in-stent restenosis.18
NaviscoreTM (iVascular, Spain)
NaviscoreTM (iVascular, Spain) is a SB with a design that combines the benefits of SB plus CB. It consists of a high-pressure balloon with 125 μm nitinol filaments. These have an axial orientation for greater crossing abilities and flexibility, and plaque modification in a 90º angle, which is associated with lower chances of perforation. The catheter hydrophilic coating improves its navigability.
In conclusion CBs and SBs are useful plaque modification devices to treat non-dilatable lesions when calcification is not very severe. Their main advantage is how easy they are to use since it is a balloon-based technique compatible with conventional angioplasty guidewires.
Very high-pressure balloons
The NC very high-pressure balloon (VHPB) OPN (SIS medical, Switzerland) is a double-layer balloon for homogeneous expansion at extremely high pressures without increasing its diameter (from 2 mm to 4 mm) with a rated burst pressure of 35 atm, although the manufacturer's testing rated burst pressure limit is 45 atm (table 5).46
Table 5. Compliance of the NC very high-pressure OPN balloon
| Pressure (atm) | NC OPN 2.0 (mm) | NC OPN 2.5 (mm) | NC OPN 3.0 (mm) | NC OPN 3.5 (mm) | NC OPN 4.0 (mm) |
|---|---|---|---|---|---|
| 10 | 2.0 | 2.5 | 3.0 | 3.5 | 4.0 |
|
| |||||
| 20 | 2.1 | 2.6 | 3.14 | 3.67 | 4.19 |
|
| |||||
| 30 | 2.18 | 2.7 | 3.29 | 3.85 | 4.37 |
|
| |||||
| 35 | 2.2 | 2.77 | 3.36 | 3.91 | 4.41 |
NC, noncompliant.
The VHPB has been used for over 10 years now and it has proven safe and effective in up to 40 atm in extremely calcified coronary artery lesions where other devices have failed or in stent underexpansion. Success rates are as high as 75% to 100% without evidence of dissection, perforation or balloon bursts in small case series.47 Compared to conventional NC balloon, it can achieve minimum luminal diameters and major acute gains with less residual stenosis in non-dilatable lesions.48
The largest registry ever conducted to this date included 326 patients with non-dilatable lesions treated with VHPB after failed NC balloon. Patients were categorized into 2 groups: those who responded to pressures between 30 atm and 40 atm, and those who responded with pressures > 40 atm. Procedural success was reached in up to 96.6% of the patients. A total of 53% of the patients responded to pressures between 30 atm and 40 atm while the remaining 47% did so to pressures > 40 atm. A total of 180 patients were treated with intracoronary imaging modalities and 106 of these showed calcifications > 270º. In this subgroup of patients, the pressured needed for optimal expansion was > 40 atm in 78.3% of the cases. Three patients (0.9%) showed coronary artery ruptures that resolved with prolonged inflation or covered stent implantation. In the 3 cases, the ruptures occurred during predilatation and were associated with balloon bursts with pressures between 30 atm and 40 atm. This is suggestive that perforations don't seem to be associated with inflation pressure but with the characteristics of the plaque or the vessel size estimate that was angiographic in the 3 cases reported.49
The ISAR-CAL trial50 was published back in 2021. It randomized 70 patients with extremely calcified coronary artery lesions and failed predilatation with NC balloon to receive a SB or a VHPB. The study primary endpoint was to compare stent expansion on the OCT. No differences were reported in the percentage of stent expansion. However, differences were seen in angiographic secondary endpoints like improved minimum luminal diameters and residual stenoses favorable to VHPB.50
Finally, chronic total coronary occlusions are the pinnacle of calcified complex lesions. In the PLACCTON trial the use of the VHPB both alone and with other plaque modification techniques was safe and effective in selected cases with chronic total coronary occlusions.51
In conclusion, the VHPB is a safe and effective alternative to treat non-dilatable calcified coronary artery lesions. Randomized clinical trials better defining this device strategy of use and the remaining plaque modification techniques are lacking.
Intracoronary lithotripsy
Intracoronary lithotripsy (ICL) system consists of a specific balloon catheter (Shockwave Medical, United States) connected to a rechargeable portable generator (figure 2D). The generator produces energy pulses that are transmitted to emitters placed inside the balloon. Pulses are emitted at a frequency of 1 per second up to a maximum of 10 pulses per application. Each balloon catheter can administer a maximum of 80 pulses. The catheter consists of a rapid-exchange semicompliant balloon with a 0.042 in crossing profile compatible with any 0.014 guidewires and 6-Fr guide catheters.
Its main indication is to treat calcified non-dilatable coronary artery lesions.
A 1:1: ratio between the vessel and balloon diameters is advised. Once it has been placed inside the lesion, the balloon inflates at 4 atm to secure proper contact between the balloon surface and the vascular wall to allow energy transfer. The balloon includes 2 emitters that receive an electric discharge from the generator that vaporizes the fluid inside generating sound waves that have a local effect. Each pulse releases the equivalent of 50 atm.
These waves run across soft tissues causing selective calcium microfractures at intima and media layer level. After pulse emission and the corresponding modification of calcium, the balloon inflates up to 6 atm to maximize luminal gain. Balloon catheter is only available at a length of 12 mm and comes in diameters of 2.5 mm, 30 mm, 3.5 mm, and 4.0 mm.52
The greatest evidence available comes from the Disrupt-CAD III trial, a prospective registry that assessed the efficacy and safety profile of ICL in 431 patients with calcified lesions. The 30-day rate of MACE (death, infarction or target lesion revascularization) was 7.8% while the rate of effectiveness (procedural success with in-stent stenosis < 50%) was 92.4%. No patients with acute myocardial infarction or complex lesions were included in this study.53 Recently, 12-month follow-up results have been published confirming rates of MACE and stent thrombosis of 13.8% and 1.1%, respectively.54
Controlled break down of coronary calcium is the basis of treatment of ICL balloons. In a OCT substudy of the Disrupt-CAD II trial after ICL calcium fractures were seen in 79% of the lesions55 compared to 67% of the lesions of the Disrupt-CAD III trial.53
Although the use of ICL balloons has become very popular worldwide, information on its safety and efficacy profile regarding its use in complex settings is still scarce (acute coronary syndrome, chronic total coronary occlusions, bifurcations or aorto-ostial lesions). As a matter of fact, its use is often limited to isolated cases or short series.52 The main limitations of this system are its reduced crossing profile in extremely calcified or tortuous stenoses and difficult use in diffuse or multivessel lesions (due to the limited number of pulses per catheter and the different caliber of target vessels).
A recent trial assessed the use of underexpanded stents due to severe coronary artery calcification and confirmed angiographic success rates of up to 73%, which is lower compared to the 75% seen in native lesions56 probably because it is more difficult to expand a calcified lesion when the stent has already been deployed. Therefore, regardless of the technique used, stenting is ill-advised until the lesion has been properly prepared. Also, the application of lithotripsy in this context, especially on freshly implanted stents, can cause structural damage to the polymer.57 Another multicenter registry proved the device was successful 92.3% of the times in this type of lesions.58 Mid- and short-term data on the safety profile of this technique are still lacking.
The combined use of ICL balloon and other plaque modification devices like RA,59 OA60 or ELCA61 has been described, and it seems like a very attractive strategy in cases where the ICL balloon cannot reach the target lesion.
In conclusion, ICL has grown exponentially in the management of non-dilatable calcified coronary artery lesions thanks to its safety and efficacy profile, and short learning curve. However, information on its use in complex scenarios and comparative results with other plaque modification techniques are still lacking.
COMBINED TECHNIQUES
There is not much evidence on the combination of devices or plaque modification techniques in extremely calcified coronary artery lesions.
The use of RA followed by CB (RotaCutting) (figure 4) or lithotripsy (RotaTripsy) (figure 6) has been described as an additional, safe, and effective technique.62-64 In both cases the concept is similar. Primarily, RA damages superficial calcium, but not the deepest calcium layers, and there are times when it is not enough for proper plaque preparation. On the other hand, CB or lithotripsy can complement the plaque modification provided by RA. However, when calcified lesions progress into very severe aortic stenosis, the target lesion can be difficult to reach with these balloons. In a combined use, RA modifies superficial calcium by creating a tunnel that the CB or lithotripsy balloon can use to move forward and, when in position, complete plaque modification. One of the differences between both techniques is that CB can contribute to breaking down the calcium layer in the absence of very severe calcification. The RotaTripsy technique59,63 can be more effective to treat extremely calcified coronary artery lesions with thick calcium layers. However, its cost is also higher. Based on a similar concept, the combination of OA plus lithotripsy has been recently described with good results.60
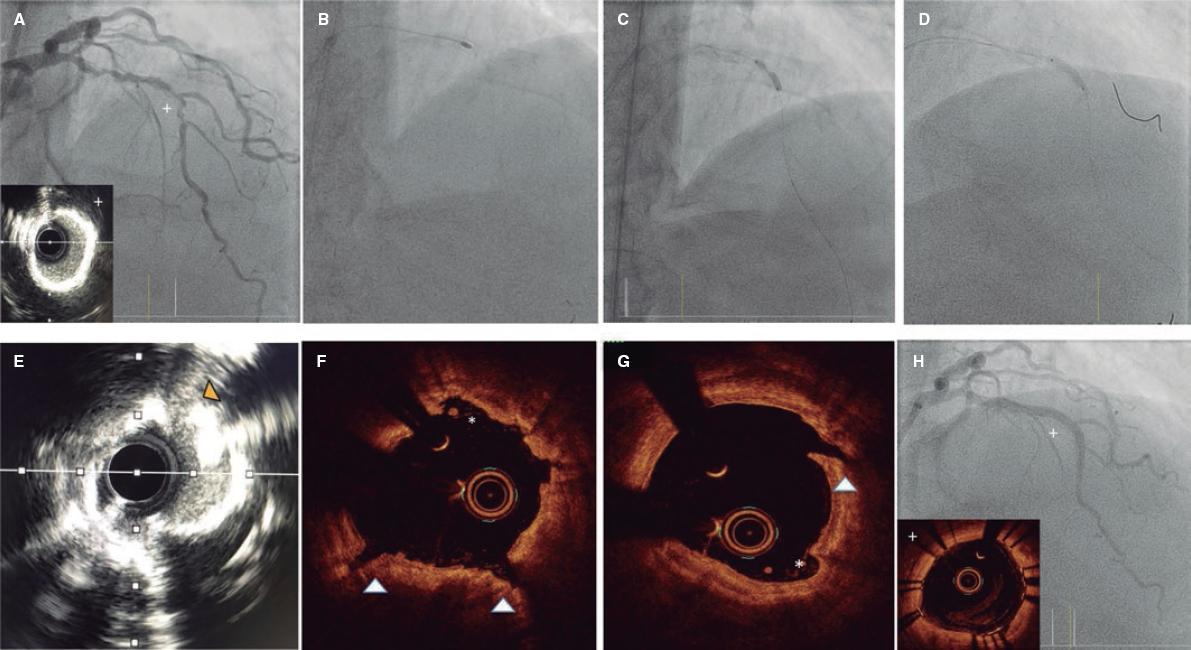
Figure 6. Rotatripsy technique. A: Extremely calcified stenosis in left anterior descending coronary artery. Intravascular ultrasound (IVUS) with 360º calcification. B: Rotational atherectomy (RA) (1.5 mm olive-shaped burr). C: 3 mm noncompliant balloon underexpansion after RA. D: Intracoronary lithotripsy with 3 mm balloon and proper expansion at 6 atm after 50 pulses. E-G: IVUS and optical coherence tomography showing the combined effect of RA plus lithotripsy with multiple zones of intimal sanding/dissection caused by the RA (asterisks), and deep and intimal fractures caused by lithotripsy. H: Final outcomes after stenting.
RA has also teamed up with ELCA (the RASER technique).65 Laser can be the only option in truly non-crossable lesions to facilitate the advancement of a microcatheter, perform the RotaWire exchange, and complete the PCI. This can also be used similarly by combining laser plus OA.
The combination of ELCA plus lithotripsy (the ELCATripsy technique) has been described for cases where RA or OA are ill-advised like nearby lesions or at freshly implanted stent level. In these cases, laser can create a tunnel through which the lithotripsy balloon can advance without the risk or damaging the freshly implanted stent.61
ALGORITHM FOR THE OPTIMAL MANAGEMENT OF CALCIFIED CORONARY ARTERY LESIONS
To select the most suitable plaque modification technique we need to become familiar with the characteristics of the different techniques available, their indications, and risks (table 6). Also, the patient's clinical profile should be taken into consideration, as well as the characteristics of the lesion, the resources available, and the operator's skills. In some complex cases, it can be reasonable to perform an ad hoc PCI for proper planning and even an angioplasty between 2 expert operators.
Table 6. Comparison of the different plaque modification techniques available
| Techniques non derived from balloon technology | Techniques derived from balloon technology | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| RA | OA | ELCA | CB | SB | VHPB | ICL | |
| Technical characteristics | |||||||
|
| |||||||
| Description of the technology involved | Diamond-coated olive-shaped burr rotating at high speed | Diamond-coated crown with elliptical rotation | Ultraviolet energy with photochemical, photothermal, and photokinetic effects | NC balloon with longitudinal microrazors | SC balloon with scoring elements on its surface | Double-layer NC balloon to allow very high-pressures | SC balloon emitting pulsatile mechanical energy |
|
| |||||||
| Mechanism of action | Differential cut/Antegrade abrasion. Additional effect from crown vibration (+) | Differential sanding/ Antegrade and retrograde abrasion. Additional effect from crown vibration (+++) | Photoablation/vaporization | Superficial cut of the plaque | Superficial cut of the plaque | Inflation at 35 atm to 40 atm | Lithotripsy/Calcium fragmentation |
|
| |||||||
| Size of devices | 1.25 mm to 2.5 mm burr | 1.25 mm crown | 0.9 mm to 2 mm catheters | 2 mm to 4 mm | 1.47 mm to 4 mm | 1.5 mm to 4 mm | 2.5 mm to 4 mm |
|
| |||||||
| Compatible GC* | 6-Fr; 1.25 mm and 1.5 mm 7-Fr; 1.75 mm 8-Fr; 2.0 mm and 2.15 mm 9-Fr; 2.25 mm and 2.38 mm 10-Fr; 2.50 mm | 6-Fr | 6-Fr: 0.9 mm and 1.4 mm 7-Fr: 1.7 mm 8-Fr: 2.0 mm | 6-Fr | 6-Fr (some with 5-Fr) | 6-Fr | 6-Fr |
|
| |||||||
| Type of compatible guidewire | 0.009 in RotaWire (0.014 in the radiopaque part) | 0.012 in ViperWire (0.014 in the radiopaque part) | Any 0.014 in guidewire | Any 0.014 in guidewire | Any 0.014 in guidewire | Any 0.014 in guidewire | Any 0.014 in guidewire |
|
| |||||||
| Type of Console/System | Small without pedal (RotaPro) | Small without pedal | Large with pedal | | | | Small without pedal |
|
| |||||||
| Indications and effects | |||||||
|
| |||||||
| Main indication | Plaque modification (non-dilatable calcified coronary lesions or only crossable through microcatheter) | Plaque modification (non-dilatable calcified coronary lesions or only crossable through microcatheter) | Plaque modification (non-crossable lesion, in-stent non-dilatable coronary lesions) | ISR | ISR | Optimization of stent expansion | Calcified plaque modification |
|
| |||||||
| Effect on intimal or deep calcium layers | Intimal | Intimal and deep | Intimal and deep | Intimal | Intimal | Intimal | Intimal and deep |
|
| |||||||
| ISR | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
|
| |||||||
| Stent underexpansion | Chronic only | Chronic only | Acute or chronic | | | Acute or chronic | Recommended in chronic only |
|
| |||||||
| Advantages | Useful in non-crossable lesion with balloon Greater availability compared to OA/ELCA | Possibility of retrograde application (useful in angulated/ostial lesions) 1 crown only for all cases (compatible with 6-Fr) | No need for specific guidewire. 0.9 mm catheter (the most common one) compatible with 6-Fr Of choice in non-crossable lesion with balloon and microcatheter It allows the use of guidewires in the side branches | Short learning curve. Compatible with 0.014 in and 6-Fr guidewires It allows the use of guidewires in the side branches. Lower cost | Short learning curve. Compatible with 0.014 in and 6-Fr guidewires It allows the use of guidewires in the side branches Lower cost | Short learning curve. Compatible with 0.014 in and 6-Fr guidewires It allows the use of guidewires in the side branches Lower cost | Short learning curve. Compatible with 0.014 in and 6-Fr guidewires It allows the use of guidewires in the side branches |
|
| |||||||
| Disadvantages | Long learning curve Need for specific guidewire Need for large French sizes for large burrs | Long learning curve Need for specific guidewire Worse crossing ability in non-crossable lesions with balloon It requires a specific lubricant contraindicated in patients allergic to egg and soybean | Intermediate learning curve Large console and need for warming up/calibration | Limited crossing ability Useless in extremely severe calcifications | Useless in extremely severe calcifications | Limited crossing ability | Limited crossing ability Limit per catheter pulses |
|
| |||||||
| Complications | |||||||
|
| |||||||
| Major perforation/dissection | Moderate | Moderate | Moderate | Low/moderate | Low/moderate | Low/moderate | Low |
|
| |||||||
| Slow/No-Flow | Moderate | Moderate | Low | Low | Low | Low | Low |
|
| |||||||
| Atrioventricular block | Moderate in dominant RCA/LCx | Moderate in dominant RCA/LCx | Low | Low | Low | Low | Low |
|
| |||||||
| Entrapment | Moderate (greater with 1.25 mm burrs and severe angulated lesions) | Low | Low | Low | Low | Low (entrapment over the guidewire is not rare; consider second parallel guidewire) | Low |
|
| |||||||
| Technical recommendations | Speeds of 135 000 rpm to 180 000 rpm Device/vessel ratio ≤ 0.6. Pecking motion Short cycles with pauses among them. Avoid angulated lesions | Speeds of 80 000 rpm to 120 000 rpm Slow continuous forward and backward motion (useful in angulated/ostial lesions) Short cycles with longer pauses among them if hemodynamic impairment Avoid antegrade access in angulated lesions | Device/vessel ratio ≤ 0.6 Slow continuous forward motion (also applicable in backward motion) Application during the injection of a saline solution Application without washing or with saline solution or contrast injection in selected cases Avoid angulated lesions | Balloon-artery ratio 1:1 Slow and gradual inflation and deflation Balloon rotation followed by repeat inflations can increase the number of incisions | Balloon-artery ratio 1:1 Slow and gradual inflation and deflation | Balloon-artery ratio 1:1 Slow and gradual inflation and deflation | Balloon-artery ratio 1:1 Optimal balloon air purge Inflation sequence at 4 atm, application of 10 pulses, and inflation at 6 atm Gradual deflation after console beeping At least 20 pulses per lesion |
CB, cutting balloon; ELCA, Excimer laser coronary angioplasty; GC, guide catheter; ICL, intracoronary lithotripsy; ISR, in-stent restenosis; LCx, left circumflex artery; MC, microcatheter; NC, noncompliant; OA, orbital atherectomy; RA, rotational atherectomy; RCA, right coronary artery; SB, scoring balloon; SC, semicompliant; VHPB, very high-pressure balloon.
*The 1.75 olive-shaped burr is compatible with some 6-Fr guide catheter models although with some level of friction (it is 0.069 in thick and requires a 0.073 in internal catheter diameter).
Current evidence available from comparative or clinical trials allowing us to select among the different plaque modification techniques available is very limited66,67 (table 7). Therefore, although several algorithms have been proposed on the type of calcium and the plaque modification technique that should be used,67 there are no clear indications in the routine clinical practice guidelines. Currently ongoing studies may bring us more in-depth information in the future.
Table 7. Main clinical trials on plaque modification techniques
| Trial (year) | Design and sample size | Type of lesion | Main results |
|---|---|---|---|
| Rotational atherectomy | |||
|
| |||
| ROTAXUS20,24 (2013) | RCA of 240 p. (120 RA: 120 ST) | Moderate to severe calcification | Successful strategy: RA, 92.5% vs ST, 83.3%; P = .03 Acute luminal gain: RA, 1.56 mm vs ST, 1.44 mm; P < .01 No significant differences regarding dissections, perforations or slow/no-reflow Stent luminal loss at 9 months: RA, 0.44 mm vs ST, 0.31 mm; P = .04 MACE at 9 months: RA, 24.2% vs ST, 28.3%; P = .46 MACE at 2 years: RA, 29.4% vs ST, 34.3%; P = .47 |
|
| |||
| PREPARE CALC21 (2018) | RCA of 200 p. AR vs MB (cutting or scoring) | Severe calcification | Successful strategy: RA, 98% vs MB, 81%; P = .0001 No significant differences regarding dissections, perforations or slow/no-reflow Luminal loss at 9 months: RA, 0.22 mm vs MB, 0.16 mm; P = .21 TLR at 9 months: RA, 2% vs MB, 7%; P = .17 No significant differences at 9 months regarding mortality or stent thrombosis |
|
| |||
| Orbital atherectomy | |||
|
| |||
| ORBIT I30 (2013) | NRPT of 50 p | Calcification (mild to severe) | Procedural success (residual stenosis <20% after stenting): 94% Rate of MACE at 6 months: 8% Dissection: 12% Perforation: 2% |
|
| |||
| ORBIT II28,31 (2014) | NRPT of 443 p | Severe calcification | Procedural success (stenosis < 50% after stenting without in-hospital MACE): 98.6% Severe dissection: 2.3% Perforation: 0.9% Slow/no-reflow: 0.2%M MACE at 30 days and 3 years: 10.4% and 23.5%, respectively |
|
| |||
| COAST29 (2020) | NRPT of 100 p | Severe calcification | Procedural success (stenosis < 50% after stenting without in-hospital MACE): 85% Dissection: 2% Perforation: 2% Slow/no-reflow: 2% MACE at 30 days and 1 year: 15% and 22.2%, respectively |
|
| |||
| ELCA | |||
|
| |||
| Fernandez et al.36 (2013) | Observational trial of 58 p | Balloon failure (non-crossable or non-dilatable lesions) treated with ELCA ± RA Calcification > moderate: 82.1% |
Procedural success (stenosis < 20% after stenting without flow-limiting dissection or type II or III perforations): 91% ELCA success isolated in 76.1%; ELCA after failed RA, 6.8% and ELCA + RA, 8.6% Only 1 successful case of RA when ELCA failed 4 procedural complications reported (1 transient slow flow, 1 side branch occlusion, and 2 perforations) |
|
| |||
| ELLEMENT37 (2014) | Observational trial of 28 p | Stent underexpansion treated with high-energy ELCA with contrast after NC balloon failure Calcification: 89.3% |
Laser success (increase ≥ 1 mm2 in SMA with IVUS or ≥ 20% MLD on the quantitative coronary angioggraphy after predilatation with the NC balloon that failed before ELCA): 96.4% Perioperative infarction: 7.1% Transient slow flow: 3.6% |
|
| |||
| LEONARDO68 (2015) | Observational trial of 100 p | Balloon failure in complex lesions Calcification: 57%. |
Procedural success (stenosis <50% after stenting): 91.7% No perforations, dissections, significant side branch occlusions, spasms or lack of flow |
|
| |||
| LAVA69 (2018) | Observational trial of 130 lesions | Non-crossable lesions with balloon: 43.8% Non-dilatable lesions with balloon: 40.8% Moderate or severe calcification: 62% ISR: 37% |
Procedural success: 88.8% (93.8% in non-dilatable lesions and 83.7% in non-crossable lesions) Perforation: 1.78% Perioperative infarction: 0.86% |
|
| |||
| Ojeda et al.34 (2020) | Observational trial of 126 lesions | Non-crossable lesions with balloon Calcification ≥ moderate: 62.7% Chronic total coronary occlusion: 46% |
Technical success (residual stenosis < 30% and TIMI grade-3 flow): 90.5% Procedural success (technical success without in-hospital adverse events): 87.3% Severe calcification associated with failed ELCA |
|
| |||
| Modified balloons (cutting or scoring balloons), and VHPB | |||
|
| |||
| ISAR-CALC50 | RCA of 74 p (VHPB vs SB) | Extremely calcified non-dilatable lesions with balloon | Stent expansion on the CTO similar compared to VHPB and SB (0.72 ± 0.12 vs 0.68 ± 0.13; P = .22) VHPB: higher increase of MLD (2.83 mm ± 0.34 mm vs 2.65 mm ± 0.36 mm; P = .03) and less stenosis (11.6% ± 4.8% vs 14.4% ± 5.6%; P = .02) No differences associated with procedural success |
|
| |||
| Intracoronary lithotripsy | |||
|
| |||
| DISRUPT CAD III53,54 | NRPT of 431 p | Severe calcification | Procedural success (residual stenosis < 50% without in-hospital MACE): 92.4% Perioperative infarction 6.8% Severe dissection: 0.3% Perforation: 0.3% Slow or no-reflow 0% TLR at 30 days: 1.3% Stent thrombosis: 0.8% MACE at 1 year 13.8% |
ELCA, Excimer laser coronary angioplasty; ISR, in-stent restenosis; IVUS, intravascular ultrasound; MACE, major adverse cardiovascular events; MB, modified balloon; MLD, minimum luminal diameter; NC, noncompliant; NRPT, non-randomized prospective trial; OCT, optimal coherence tomography, p, patients; QCA, quantitative coronary angiography; RA, rotational atherectomy; RCA, randomized clinical trial; SB, scoring balloon; SMA, stent minimal area; ST, standard therapy; TIMI, Thrombolysis in Myocardial Infarction; TLR, target lesion revascularization; VHPB, very high-pressure balloon.
In cases of mild angiographic calcification and proper balloon expansion, further plaque preparation prior to stenting may not be required. However, when angiographic calcification is moderate or severe, the use of intracoronary imaging modalities is advised for their great utility to plan the procedure and optimize results (figure 7).
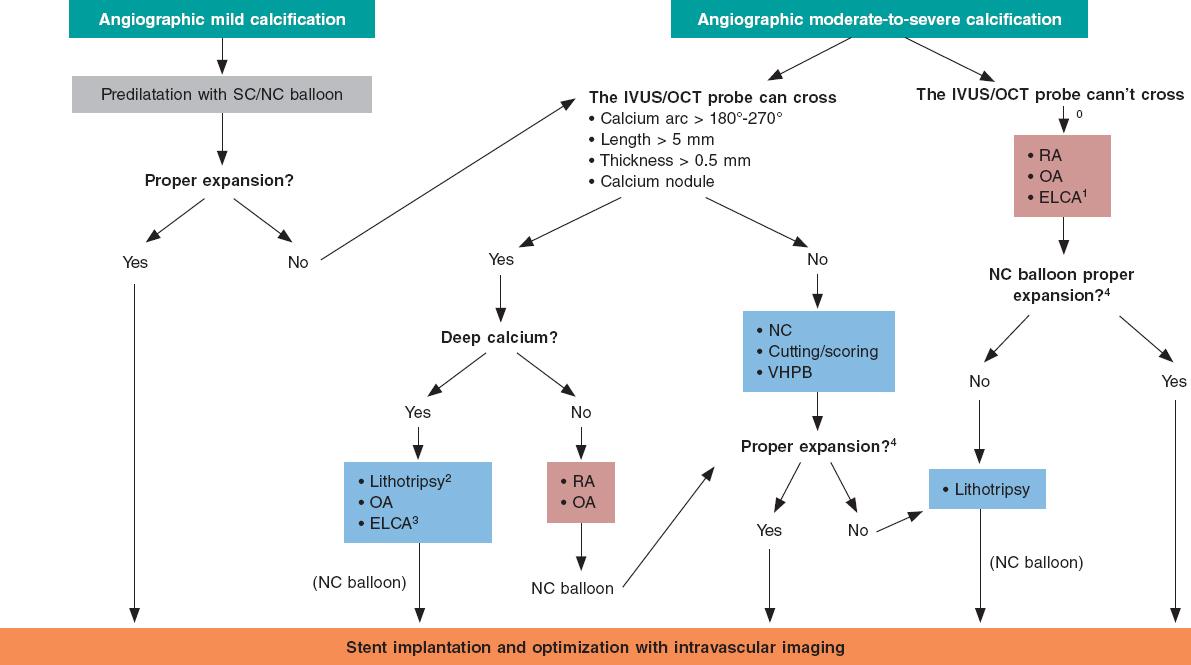
Figure 7. Central figure. Calcified plaque modification algorithm. ELCA, Excimer laser coronary angioplasty; NC, noncompliant; OA, orbital atherectomy; RA, rotational atherectomy; SC, semicompliant; VHPB, very high-pressure balloon. 0 Predilatation with low-profile balloons can be attempted. At times, this allows early assessment with intravascular imaging tools. 1 Of choice if microcatheter is unable to cross. 2 If lithotripsy balloon is unable to cross, predilatation can be attempted with balloon or combination of other techniques (Rotatripsy, Elcatripsy, Orbital-tripsy). 3 Currently, lithotripsy is preferred in the presence of acute stent underexpansion. 4 In addition to NC balloon final angiographic expansion, intracoronary imaging are useful to confirm the effect of the techniques used on plaque modification.
Overall, it is useful to apply the rule of 5: lesions where calcium occupies < 50% of arc circumference (180º), does not extend longitudinally > 5 mm, and thickness is not > 0.5 mm can be properly treated with high-pressure or modified balloons (CB or SB).
If these criteria are met or calcium nodules are spotted further advanced plaque modification techniques should be used. In addition to circumferential and longitudinal spread, and thickness, calcium depth is important as well since some techniques like RA act basically on the superficial and not on the deep portion of calcium plaque.
Lesions with extremely severe calcifications so stenotic that cannot be crossed with the IVUS or OCT probe probably need RA/OA or laser (that can be of choice if the lesion is non-crossable not even with a microcatheter to allow specific RA/OA guidewire exchange). Another alternative is to try predilatation with low-profile balloons that often allow early assessments with intravascular coronary imaging to guide the decision-making process as already described.
Balloon expansion after using these techniques will guide us on proper plaque preparation. Also, intracoronary images are very useful to confirm proper calcium modification to allow stent expansion. The effects of different techniques like the presence of fractures (with balloon or lithotripsy), superficial calcium sanding (with RA) or both effects (with OA)70 can be visible when intracoronary imaging modalities are used (figure 6). After the use of ELCA, superficial and deep fractures have been described. However, effects may not be visible on the OCT and, same as it happens with ICL, that does not mean that the plaque has not been modified.
Based on the type of lesion and effects caused by these techniques, the combination of 1 or more of these techniques can be necessary to secure optimal stenting and favorable clinical outcomes.
CONCLUSIONS
Coronary artery calcification is probably the greatest determinant of poor PCI outcomes and incomplete percutaneous revascularizations, and is associated with higher rates of adverse events. Intracoronary imaging modalities play a key role in the understanding of calcified coronary artery lesions, help us select the plaque modification technique we'll eventually use, and optimize the PCI results. Knowing the different plaque modification techniques available is essential for the optimal management of calcified coronary artery lesions. Until comparative trials among techniques are conducted, it seems reasonable to combine them depending on the type of lesion. In addition, there are situations in which techniques should be combined to secure optimal stenting and the most favorable clinical outcomes.
AUTHORS' CONTRIBUTIONS
Manuscript drafting: A. Jurado-Román, A. Gómez-Menchero, N. Gonzalo, J. Martín-Moreiras, R. Ocaranza, Soledad Ojeda, J. Palazuelos, O. Rodríguez-Leor, P. Salinas, B. Vaquerizo, X. Freixa, and A.B. Cid-Álvarez. Design, coordination, review process of the final version, and manuscript submission: A. Jurado-Román.
REFERENCES
1. Mori H, Torii S, Kutyna M, Sakamoto A, Finn AV, Virmani R. Coronary Artery Calcification and its Progression:What Does it Really Mean?JACC Cardiovasc Imaging. 2018;11:127-142. [ Links ]
2. Wang X, Matsumura M, Mintz GS, et al. In Vivo Calcium Detection by Comparing Optical Coherence Tomography, Intravascular Ultrasound, and Angiography. JACC Cardiovasc Imaging. 2017;10:869-879. [ Links ]
3. Copeland-Halperin RS, Baber U, Aquino M, et al. Prevalence, correlates, and impact of coronary calcification on adverse events following PCI with newer-generation DES:Findings from a large multiethnic registry. Catheter Cardiovasc Interv. 2018;91:859-866. [ Links ]
4. Sharma SK, Bolduan RW, Patel MR, et al. Impact of calcification on percutaneous coronary intervention:MACE-Trial 1-year results. Catheter Cardiovasc Interv. 2019;94:187-194. [ Links ]
5. Fujino A, Mintz GS, Matsumura M, et al. A new optical coherence tomography-based calcium scoring system to predict stent underexpansion. EuroIntervention. 2018;13:e2182-e2189. [ Links ]
6. Serruys PW, Morice MC, Kappetein AP, et al. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med. 2009;360:961-972. [ Links ]
7. Kawashima H, Serruys PW, Hara H, et al. 10-Year All-Cause Mortality Following Percutaneous or Surgical Revascularization in Patients With Heavy Calcification. JACC Cardiovasc Interv. 2022;15:193-204. [ Links ]
8. Hendry C, Fraser D, Eichhofer J, et al. Coronary perforation in the drug-eluting stent era:incidence, risk factors, management and outcome:the UK experience. EuroIntervention. 2012;8:79-86. [ Links ]
9. Mintz GS, Popma JJ, Pichard AD, et al. Patterns of calcification in coronary artery disease. A statistical analysis of intravascular ultrasound and coronary angiography in 1155 lesions. Circulation. 1995;91:1959-1965. [ Links ]
10. Zhang M, Matsumura M, Usui E, et al. Intravascular Ultrasound-Derived Calcium Score to Predict Stent Expansion in Severely Calcified Lesions. Circ Cardiovasc Interv. 2021;e010296. [ Links ]
11. Stefanini GG, Alfonso F, Barbato E, et al. Management of Myocardial Revascularization Failure:An Expert Consensus Document of the EAPCI. EuroIntervention. 2020;16:e875-e890. [ Links ]
12. Räber L, Mintz GS, Koskinas KC, et al. Clinical use of intracoronary imaging. Part 1:guidance and optimization of coronary interventions. An expert consensus document of the European Association of Percutaneous Cardiovascular Interventions. EuroIntervention. 2018;14:656-677. [ Links ]
13. Cubero-Gallego H, Tizón-Marcos H, Vaquerizo B. Opciones actuales para el tratamiento de las lesiones calcificadas. REC Interv Cardiol. 2020;2:129-139. [ Links ]
14. Hellig F, Pandie S, Barbato E, Colombo A, Heyndrickx JR. Rotational atherectomy. En:PCR - EAPCI Textbook 2015, Part III. Europa Digital &Publishing;2015. [ Links ]
15. Sharma SK, Tomey MI, Teirstein PS, et al. North American Expert Review of Rotational Atherectomy. Circ Cardiovasc Interv. 2019;12:e007448. [ Links ]
16. Barbato E, CarriéD, Dardas P, et al. European expert consensus on rotational atherectomy. EuroIntervention. 2015;11:30-36. [ Links ]
17. De Maria GL, Scarsini R, Banning AP. Management of Calcific Coronary Artery Lesions:Is it Time to Change Our Interventional Therapeutic Approach?JACC Cardiovasc Interv. 2019;12:1465-1478. [ Links ]
18. Bittl JA, Chew DP, Topol EJ, Kong DF, Califf RM. Meta-analysis of randomized trials of percutaneous transluminal coronary angioplasty versus atherectomy, cutting balloon atherotomy, or laser angioplasty. J Am Coll Cardiol. 2004;43:936-942. [ Links ]
19. Safian RD, Feldman T, Muller DW, et al. Coronary angioplasty and Rotablator atherectomy trial (CARAT):immediate and late results of a prospective multicenter randomized trial. Catheter Cardiovasc Interv. 2001;53:213-220. [ Links ]
20. Abdel-Wahab M, Richardt G, Joachim Büttner H, et al. High-speed rotational atherectomy before paclitaxel-eluting stent implantation in complex calcified coronary lesions:the randomized ROTAXUS (Rotational Atherectomy Prior to Taxus Stent Treatment for Complex Native Coronary Artery Disease) trial. JACC Cardiovasc Interv. 2013;6:10-19. [ Links ]
21. Abdel-Wahab M, Toelg R, Byrne RA, et al. High-Speed Rotational Atherectomy Versus Modified Balloons Prior to Drug-Eluting Stent Implantation in Severely Calcified Coronary Lesions. Circ Cardiovasc Interv. 2018;11:e007415. [ Links ]
22. Iannaccone M, Piazza F, Boccuzzi GG, et al. ROTational AThErectomy in acute coronary syndrome:early and midterm outcomes from a multicentre registry. EuroIntervention. 2016;12:1457-1464. [ Links ]
23. Kawamoto H, Latib A, Ruparelia N, et al. In-hospital and midterm clinical outcomes of rotational atherectomy followed by stent implantation:the ROTATE multicentre registry. EuroIntervention. 2016;12:1448-1456. [ Links ]
24. de Waha S, Allali A, Büttner HJ, et al. Rotational atherectomy before paclitaxel-eluting stent implantation in complex calcified coronary lesions:Two-year clinical outcome of the randomized ROTAXUS trial. Catheter Cardiovasc Interv. 2016;87:691-700. [ Links ]
25. Cardiovascular System (CSI). Diamondback 360 Coronary Orbital Atherectomy System. Disponible en:https://csi360.com/diamondback-coronary-orbital-atherectomy-system/. Consultado 27 sep 2022. [ Links ]
26. Shlofmitz E, Shlofmitz R, Lee MS. Orbital Atherectomy:A Comprehensive Review. Interv Cardiol Clin. 2019;8:161-171. [ Links ]
27. Yamamoto MH, Maehara A, Karimi Galougahi K, et al. Mechanisms of Orbital Versus Rotational Atherectomy Plaque Modification in Severely Calcified Lesions Assessed by Optical Coherence Tomography. JACC Cardiovasc Interv. 2017;10:2584-2586. [ Links ]
28. Chambers JW, Feldman RL, Himmelstein SI, et al. Pivotal trial to evaluate the safety and efficacy of the orbital atherectomy system in treating de novo, severely calcified coronary lesions (ORBIT II). JACC Cardiovasc Interv. 2014;7:510-518. [ Links ]
29. Redfors B, Sharma SK, Saito S, et al. Novel Micro Crown Orbital Atherectomy for Severe Lesion Calcification:Coronary Orbital Atherectomy System Study (COAST). Circ Cardiovasc Interv. 2020;13:e008993. [ Links ]
30. Parikh K, Chandra P, Choksi N, Khanna P, Chambers J. Safety and feasibility of orbital atherectomy for the treatment of calcified coronary lesions:the ORBIT I trial. Catheter Cardiovasc Interv. 2013;81:1134-1139. [ Links ]
31. Lee M, Généreux P, Shlofmitz R, et al. Orbital atherectomy for treating de novo, severely calcified coronary lesions:3-year results of the pivotal ORBIT II trial. Cardiovasc Revasc Med. 2017;18:261-264. [ Links ]
32. Tsutsui RS, Sammour Y, Kalra A, et al. Excimer Laser Atherectomy in Percutaneous Coronary Intervention:A Contemporary Review. Cardiovasc Revasc Med. 2021;25:75-85. [ Links ]
33. Golino L, Caiazzo G, CalabròP, et al. Excimer laser technology in percutaneous coronary interventions:Cardiovascular laser society's position paper. Int J Cardiol. 2022;350:19-26. [ Links ]
34. Ojeda S, Azzalini L, Suárez de Lezo J, et al. Excimer laser coronary atherectomy for uncrossable coronary lesions. A multicenter registry. Catheter Cardiovasc Interv. 2021;98:1241-1249. [ Links ]
35. Mintz GS, Kovach JA, Javier SP, et al. Mechanisms of lumen enlargement after excimer laser coronary angioplasty. An intravascular ultrasound study. Circulation. 1995;92:3408-3414. [ Links ]
36. Fernandez JP, Hobson AR, McKenzie D, et al. Beyond the balloon:excimer coronary laser atherectomy used alone or in combination with rotational atherectomy in the treatment of chronic total occlusions, non-crossable and non-expansible coronary lesions. EuroIntervention. 2013;9:243-250. [ Links ]
37. Latib A, Takagi K, Chizzola G, et al. Excimer Laser LEsion modification to expand non-dilatable stents:the ELLEMENT registry. Cardiovasc Revasc Med. 2014;15:8-12. [ Links ]
38. Sintek M, Coverstone E, Bach R, et al. Excimer Laser Coronary Angioplasty in Coronary Lesions:Use and Safety From the NCDR/CATH PCI Registry. Circ Cardiovasc Interv. 2021;14(7):e010061. [ Links ]
39. Protty MB, Hussain HI, Gallagher S, et al. Excimer laser coronary atherectomy during complex PCI:An analysis of 1,471 laser cases from the British Cardiovascular Intervention Society database. Catheter Cardiovasc Interv. 2021;97:E653-E660. [ Links ]
40. Unterberg C, Buchwald AB, Barath P, Schmidt T, Kreuzer H, Wiegand V. Cutting balloon coronary angioplasty -- initial clinical experience. Clin Cardiol. 1993;16:660-664. [ Links ]
41. Barath P, Fishbein MC, Vari S, Forrester JS. Cutting balloon:a novel approach to percutaneous angioplasty. Am J Cardiol. 1991;68:1249-1252. [ Links ]
42. Bonaventura K, Schwefer M, Yusof AKM, et al. Systematic Scoring Balloon Lesion Preparation for Drug-Coated Balloon Angioplasty in Clinical Routine:Results of the PASSWORD Observational Study. Adv Ther. 2020;37:2210-2223. [ Links ]
43. Okura H, Hayase M, Shimodozono S, et al. Mechanisms of acute lumen gain following cutting balloon angioplasty in calcified and noncalcified lesions:an intravascular ultrasound study. Catheter Cardiovasc Interv. 2002;57:429-436. [ Links ]
44. Barbato E, Shlofmitz E, Milkas A, Shlofmitz R, Azzalini L, Colombo A. State of the art:evolving concepts in the treatment of heavily calcified and undilatable coronary stenoses - from debulking to plaque modification, a 40-year-long journey. EuroIntervention. 2017;13:696-705. [ Links ]
45. de Ribamar Costa JJ, Mintz GS, Carlier SG, et al. Nonrandomized comparison of coronary stenting under intravascular ultrasound guidance of direct stenting without predilation versus conventional predilation with a semi-compliant balloon versus predilation with a new scoring balloon. Am J Cardiol. 2007;100:812-817. [ Links ]
46. Felekos I, Karamasis GV, Pavlidis AN. When everything else fails:High-pressure balloon for undilatable lesions. Cardiovasc Revasc Med. 2018;19:306-313. [ Links ]
47. Díaz JF, Gómez-Menchero A, Cardenal R, Sánchez-González C, Sanghvi A. Extremely high-pressure dilation with a new noncompliant balloon. Tex Heart Inst J. 2012;39:635-638. [ Links ]
48. Secco GG, Ghione M, Mattesini A, et al. Very high-pressure dilatation for undilatable coronary lesions:indications and results with a new dedicated balloon. EuroIntervention. 2016;12:359-365. [ Links ]
49. Secco GG, Buettner A, Parisi R, et al. Clinical Experience with Very High-Pressure Dilatation for Resistant Coronary Lesions. Cardiovasc Revasc Med. 2019;20:1083-1087. [ Links ]
50. Rheude T, Rai H, Richardt G, et al. Super high-pressure balloon versus scoring balloon to prepare severely calcified coronary lesions:the ISAR-CALC randomised trial. EuroIntervention. 2021;17:481-488. [ Links ]
51. Delgado-Arana JR, Rumoroso JR, Regueiro A, et al. Plaque modification in calcified chronic total occlusions:the PLACCTON study. Rev Esp Cardiol. 2022;75:213-222. [ Links ]
52. Vilalta del Olmo V, Rodríguez-Leor O, Redondo A, et al. Intracoronary lithotripsy in a high-risk real-world population. First experience in severely calcified, complex coronary lesions. REC Interv Cardiol. 2020;2:76-81. [ Links ]
53. Hill JM, Kereiakes DJ, Shlofmitz RA, et al. Intravascular Lithotripsy for Treatment of Severely Calcified Coronary Artery Disease. J Am Coll Cardiol. 2020;76:2635-2646. [ Links ]
54. Kereiakes DJ, Hill JM, Shlofmitz RA, et al. Intravascular Lithotripsy for Treatment of Severely Calcified Coronary Lesions:1-Year Results From the Disrupt CAD III Study. J Soc Cardiovasc Angiogr Interv. 2022. https://doi.org/10.1016/j.jscai.2021.100001. [ Links ]
55. Ali ZA, Nef H, Escaned J, et al. Safety and Effectiveness of Coronary Intravascular Lithotripsy for Treatment of Severely Calcified Coronary Stenoses:The Disrupt CAD II Study. Circ Cardiovasc Interv. 2019;12:e008434. [ Links ]
56. Pham V, Bonnet M, Varenne O, et al. In-stent use of Intravascular Coronary Lithotripsy for restenosis and stent underexpansion, a multicenter experience. Can J Cardiol. 2022;10:1474-1475. [ Links ]
57. Achim A, Alampi C, Krivoshei L, Leibundgut G. In vitro effect of intravascular lithotripsy on the polymer of a drug-eluting stent. EuroIntervention. 2022;18:e333-e334. [ Links ]
58. Tovar Forero MN, Sardella G, Salvi N, et al. Coronary lithotripsy for the treatment of underexpanded stents;the international &multicentre CRUNCH registry. EuroIntervention. 2022;18:574-581. [ Links ]
59. Gonzálvez-García A, Jiménez-Valero S, Galeote G, Moreno R, López de SáE, Jurado-Román A. RotaTripsy:Combination of Rotational Atherectomy and Intravascular Lithotripsy in Heavily Calcified Coronary Lesions:A Case Series. Cardiovasc Revasc Med. 2022;35:179-184. [ Links ]
60. Yarusi BB, Jagadeesan VS, Hussain S, et al. Combined Coronary Orbital Atherectomy and Intravascular Lithotripsy for the Treatment of Severely Calcified Coronary Stenoses:The First Case Series. J Invasive Cardiol. 2022;34:E210-E217. [ Links ]
61. Jurado-Román A, García A, Moreno R. ELCA-Tripsy:Combination of Laser and Lithotripsy for Severely Calcified Lesions. J Invasive Cardiol. 2021;33:E754-E755. [ Links ]
62. Amemiya K, Yamamoto MH, Maehara A, et al. Effect of cutting balloon after rotational atherectomy in severely calcified coronary artery lesions as assessed by optical coherence tomography. Catheter Cardiovasc Interv. 2019;94:936-944. [ Links ]
63. Jurado-Román A, Gonzálvez A, Galeote G, Jiménez-Valero S, Moreno R. RotaTripsy:Combination of Rotational Atherectomy and Intravascular Lithotripsy for the Treatment of Severely Calcified Lesions. JACC Cardiovasc Interv. 2019;12:e127-e129. [ Links ]
64. Chen G, Zrenner B, Pyxaras SA. Combined Rotational Atherectomy and Intravascular Lithotripsy for the Treatment of Severely Calcified in-Stent Neoatherosclerosis:A Mini-Review. Cardiovasc Revasc Med. 2019;20:819-821. [ Links ]
65. Protty MB, Gallagher S, Farooq V, et al. Combined use of rotational and excimer lASER coronary atherectomy (RASER) during complex coronary angioplasty - An analysis of cases (2006-2016) from the British Cardiovascular Intervention Society database. Catheter Cardiovasc Interv. 2021;97:E911-E918. [ Links ]
66. McInerney A, Escaned J, Gonzalo N. Calcified coronary artery disease:pathophysiology, intracoronary imaging assessment, and plaque modification techniques. REC Interv Cardiol. 2022;4:216-227. [ Links ]
67. Bulluck H, McEntegart M. Contemporary tools and devices for coronary calcium modification. JRSM Cardiovasc Dis. 2022. https://doi.org/10.1177/20480040221089760. [ Links ]
68. Ambrosini V, Sorropago G, Laurenzano E, et al. Early outcome of high energy Laser (Excimer) facilitated coronary angioplasty ON hARD and complex calcified and balloOn-resistant coronary lesions:LEONARDO Study. Cardiovasc Revasc Med. 2015;16:141-146. [ Links ]
69. Karacsonyi J, Armstrong EJ, Truong HTD, et al. Contemporary Use of Laser During Percutaneous Coronary Interventions:Insights from the Laser Veterans Affairs (LAVA) Multicenter Registry. J Invasive Cardiol. 2018;30:195-201. [ Links ]
70. Yamamoto MH, Maehara A, Kim SS, et al. Effect of orbital atherectomy in calcified coronary artery lesions as assessed by optical coherence tomography. Catheter Cardiovasc Interv. 2019;93:1211-1218. [ Links ]
Received: August 06, 2022; Accepted: September 30, 2022











 texto em
texto em 


