Meu SciELO
Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
REC: Interventional Cardiology
versão On-line ISSN 2604-7276versão impressa ISSN 2604-7306
REC Interv Cardiol ES vol.5 no.2 Madrid Abr./Jun. 2023 Epub 18-Mar-2024
https://dx.doi.org/10.24875/recic.m22000348
ORIGINAL ARTICLES
Transcatheter aortic valve implantation using Evolut PRO versus SAPIEN 3 valves: a randomized comparative trial
aCardiology Department, Assiut University Heart Hospital, Assiut University, Assiut, Egipto
bCardiology Department, Duisburg Heart Center, Duisburg, Alemania
cCardiac Surgery Department, Duisburg Heart Center, Duisburg, Alemania
Abbreviations
AS: |
aortic stenosis. |
PVL: |
paravalvular leak. |
TAVI: |
transcatheter aortic valve implantation. |
VARC: |
Valve Academic Research Consortium. |
INTRODUCTION
Over the past decade, the self-expandable CoreValve (Medtronic Ltd, United States) and the balloon-expandable SAPIEN valve (Edwards Lifesciences Ltd, United States) were the valves most commonly used for transcatheter aortic valve implantation (TAVI).1
There are few studies comparing Evolut PRO (Medtronic Ldt, United States) vs SAPIEN 3 (Edwards Lifesciences Ltd, United States), like the SMART trial for small aortic annulI2 and the ALSTER-TAVI all-comers registry.3 However, comparative randomized clinical trials are lacking. Therefore, we designed the present randomized study to provide a head-to-head comparison between these 2 valves regarding procedural data and in-hospital outcomes especially paravalvular leak (PVL). Although the transcatheter heart valves used in this trial are not the latest generation valves of the CoreValve and SAPIEN families (currently, the Evolut-Pro plus and the SAPIEN Ultra), this is the first randomized clinical trial to compare a self-expanding valve with an outer skirt to a balloon expandable valve (with an outer skirt too).
METHODS
Study population
A total of 110 consecutive patients with severe symptomatic aortic stenosis eligible for TAVI were randomly assigned to receive the Evolut PRO valve (51 patients) or the SAPIEN 3 valve (59 patients) at Duisburg Heart Center, Duisburg, Germany, from December 2019 through May 2020. All patients undergoing TAVI for severe aortic stenosis with the SAPIEN 3 and the Evolut PRO via femoral access were included. Patients who underwent TAVI with other valve types like transapically implanted aortic valves, bicuspid aortic valves, and valve-in-surgical-bioprosthesis implantation were excluded. All procedures were performed after obtaining the patients' written informed consent and in compliance with the national research committee ethical standards.
Procedural aspects
TAVIs were performed under local anesthesia and conscious sedation. Femoral cutdown was used in all the patients. Annular dimensions were obtained by transesophageal echocardiography-guided balloon sizing during the procedure. With this technique we were able to measure annuli with transesophageal echocardiography and then choose a balloon equal to annular size. Balloon inflation during rapid pacing and aortic angiography were performed with 3 different possibilities in mind a) the balloon completely fills the annulus with no para-balloon leak or waisting indicative that annular size equals the balloon size; b) para-balloon leak is indicative that the annulus is 1 mm to 2 mm larger than balloon size; c) balloon waisting is indicative that the annulus is 1 mm to 2 mm smaller than balloon size.4 Valve type (SAPIEN 3 or Evolut PRO) was randomly selected (using simple randomization method; Monday cases for Evolut and Thursday cases for SAPIEN). Valve size was based on the annular dimensions as suggested by the manufacturers. Based on annular diameter and the diameter of the valve finally selected, a so-called cover index was calculated.5
Endpoints
Our primary endpoints were PVL, in-hospital mortality, and the rate of permanent pacemaker implantation (PPI). The study secondary endpoints were valve embolization, need for a second valve, aortic rupture or dissection, stroke or transient ischemic attack, major vascular complications, and acute kidney injury. Endpoints were defined according to the Valve Academic Research Consortium-2 (VARC-2) definitions.6
PVL assessment
Immediate PVL was semi-quantitatively assessed using Seller's criteria 7: 0/4 (absent); 1/4 (mild); 2/4 (moderate); 3/4 (moderate- to-severe); and 4/4 (severe).7 Transvalvular pressure gradients were obtained invasively using the pullback method. Aortic regurgitation index (AR index) was calculated.8
In case of significant PVL ≥ grade II, if needed, balloon postdilatation using the VACS III or NUCLEUS balloon (NuMED, United States) or else implantation of second valve was used. TTE was performed at discharge to quantify PVL according to the main VARC-2 criteria.9
Assessment of anatomical factors possibly associated with PVL
The following measurements were supported by Philips software (Philips Medical, The Netherlands): the left ventricular outflow tract/ascending aorta (LVOT/AAo) angle was defined as the angle between the axis of the first 4 cm of the ascending aorta (contact surface with the upper part of the prosthesis), and the LVOT axis (the valve landing zone) indicated by a line perpendicular to the plane of the aortic valve annulus).10
Aortic angulation (AA) angle was defined as the angle between the horizontal plane and the plane of aortic annulus.11 We categorized it into < 48° and ≥ 48°.12
Both angles were measured in the optimal fluoroscopic deployment position with all 3 coronary cusps in the same plane (figure 1). Valve implantation depth was assessed in the deployment position on the fluoroscopy from the native aortic annular margin on the side of both the non-coronary cusp (NCC) and left coronary cusp to the proximal edge of the deployed valve on the corresponding side13 (figure 2). Aortic root calcification was fluoroscopically assessed as inexistent, mild (small, isolated calcification spots), moderate (multiple large calcification spots) or severe (extensive calcification).13 Presence or absence of LVOT and mitral annular calcification were also noted.
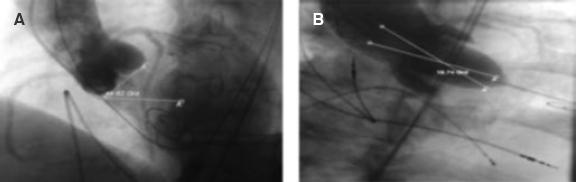
Figure 1. Measurement of different angles. AA, aortic angulation (49.62º). B: LVOT/AAo angle (18.74º). AAo, ascending aorta; LVOT, left ventricular outflow tract.
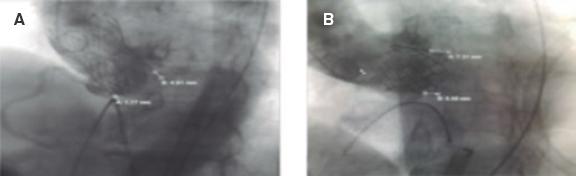
Figure 2. Measurement of implantation depth in the Evolut PRO value. A: [A = 1.17 mm associated with the NCC, and B = 4.91 mm associated with the LCC], and SAPIEN 3. B: [A = 5.65 mm associated with the NCC, and B = 7.31 mm associated with the LCC]. Note high implantation associated with the NCC due to increased LVOT/AAo angle in the Evolut PRO (A) but not in the SAPIEN 3 valve (B). AAo, ascending aorta; LCC, left coronary cusp; LVOT, left ventricular outflow tract; NCC, non-coronary cusp.
Statistical analysis
Data was collected and analyzed using the SPSS (Statistical Software Package for the Social Sciences, version 20, IBM, and Armonk, United States). Continuous data was expressed as mean ± SD or median (range). Nominal data was expressed as frequency (percentage). For the comparison of nominal and continuous data, the chi-square test and the Student's t test were used, respectively. Pearson correlation was used to assess the correlation between implantation depth with LVOT and AA angles based on the type of valve. The level of confidence was kept at 95% and hence, P values < .05 were considered statistically significant. Univariable logistic regression analysis was performed for predictors of significant PVL. ROC analysis was performed for the optimum cut-off value of the LVOT/AAo angle for the outcome of significant PVL.
Regarding sample size, assuming a 1:1 ratio in treatment assignments and an estimated rate of a composite primary endpoint (PVL, in-hospital mortality and rate of pacemaker implantation) of 8% in each study group, we estimated that a total of 52 patients were required in each group for the study to reach an 80% statistical power % at a 1-sided alpha level of 0.05
RESULTS
Baseline characteristics
A total of 110 consecutive patients with severe symptomatic aortic stenosis eligible for TAVI were randomly assigned to receive the Evolut PRO (51 patients) or the SAPIEN 3 valve (59 patients). There was no crossover between both study arms. Baseline clinical characteristics were comparable between both types of valves apart from a significantly higher body mass index among SAPIEN 3 patients and a significantly high baseline right bundle branch block in the SAPIEN group (table 1).
Table 1. Patient characteristics associated with the type of valve implanted
| Type of valve | P | ||
|---|---|---|---|
|
| |||
| Evolut PRO (N = 51) | SAPIEN 3 (N = 59) | ||
| Age (years) | 82.6 ± 6.4 | 81.2 ± 5.8 | .22 |
|
| |||
| Sex | .39 | ||
|
| |||
| Male | 54.9 | 59.3 | |
|
| |||
| Female | 45.1 | 40.7 | |
|
| |||
| Body mass index (kg/m2) | 26.4± 4.7 | 28.7 ± 4.7 | .01a |
|
| |||
| Body surface area (m2) | 1.9 ± 0.4 | 1.9 ± 0.2 | .08 |
|
| |||
| Peripheral artery disease | 11.8 | 6.8 | .28 |
|
| |||
| Hypertension | 76.5 | 83.1 | .26 |
|
| |||
| Diabetes mellitus | 29.4 | 37.3 | .25 |
|
| |||
| Ischemic heart disease | 62.0 | 45.8 | .06 |
|
| |||
| Previous revascularization (PCI/CABG) | 41.2 | 37.3 | .53 |
|
| |||
| Previous history of stroke | 5.9 | 5.1 | .58 |
|
| |||
| Previous pacemaker | 9.8 | 6.8 | .40 |
|
| |||
| Chronic chest disease | 9.8 | 23.7 | .31 |
|
| |||
| NYHA class | .09 | ||
|
| |||
| II | 13.7 | 15.3 | |
|
| |||
| III | 86.3 | 78.0 | |
|
| |||
| IV | 0.0 | 6.8 | |
|
| |||
| STS score | 3.8 ± 2.6 | 3.5 ± 2.2 | .51 |
|
| |||
| STS class (%) | .65 | ||
|
| |||
| Low (< 4%) | 58.8 | 66.1 | |
|
| |||
| Intermediate (4% to 8%) | 35.3 | 27.1 | |
|
| |||
| High (> 8%) | 5.9 | 6.8 | |
|
| |||
| ECG findings | .95 | ||
|
| |||
| Sinus | 43.1 | 45.8 | |
|
| |||
| Paced | 7.8 | 6.8 | |
|
| |||
| Atrial fibrillation | 49.0 | 47.5 | |
|
| |||
| Total preoperative conduction defects | 19.6 | 22.0 | .47 |
|
| |||
| Baseline RBBB | 0.0 | 16.9 | .001b |
Unless otherwise indicated, data are expressed as no. (%). Preoperative conduction defects included atrioventricular block, intraventricular conduction delay, left anterior hemiblock, left bundle branch block, and RBBB. CABG, coronary artery bypass graft; COPD, chronic obstructive pulmonary disease; OSAS, obstructive sleep apnea syndrome; PCI, percutaneous coronary intervention; RBBB, right bundle branch block; STS, Society of Thoracic Surgery risk score.
aSignificant P values.
bHighly significant P values.
Echocardiographic and fluoroscopic findings
The baseline echocardiographic and fluoroscopic findings of both groups were comparable (table 2).
Table 2. Echocardiographic and fluoroscopic data among the different study groups
| Type of valve | P | ||
|---|---|---|---|
|
| |||
| Evolut PRO (N = 51) | SAPIEN 3 (N = 59) | ||
| Mean PG (mmHg) | 42.3 ± 7.7 | 42.8 ± 9.9 | .78 |
|
| |||
| Maximum PG (mmHg) | 68.5 ± 10.5 | 67.3 ± 12.0 | .56 |
|
| |||
| Aortic valve area (mm) | 0.9 ± 0.7 | 0.9 ± 0.2 | .46 |
|
| |||
| Ejection fraction (%) | |||
|
| |||
| Class of ejection fraction | 48.4 ± 11.7 | 50.9 ± 11.7 | .25 .76 |
|
| |||
| Preserved (> 50%) | 62.7 | 67.8 | |
|
| |||
| Mildly impaired (40% to 50%) | 17.6 | 15.3 | |
|
| |||
| Moderately impaired (30% to 40%) | 9.8 | 11.9 | |
|
| |||
| Severely impaired (< 30%) | 9.8 | 5.1 | |
|
| |||
| Flow gradient (%) | |||
|
| |||
| HFHG | 74.5 | 71.2 | .91 |
|
| |||
| LFLG/Impaired EF | 19.6 | 22.0 | |
|
| |||
| LFLG/Preserved EF | 5.9 | 6.8 | |
|
| |||
| Aortic measurements (by TEE) | |||
|
| |||
| Aortic valve area (mm) | 0.7 ± 0.1 | 0.7 ± 0.2 | .22 |
|
| |||
| Annulus (mm) | 23.8 ± 2.1 | 24.5 ± 1.9 | .07 |
|
| |||
| LVOT (mm) | 21.1 ± 2.1 | 21.6 ± 2.4 | .26 |
|
| |||
| Sinus of Valsalva (mm) | 30.7 ± 3.6 | 31.2 ± 3.8 | .45 |
|
| |||
| Sinotubular junction (mm) | 25.9 ± 3.1 | 26.4 ± 3.5 | .37 |
|
| |||
| Ascending aorta (mm) | 33.3 ± 5.9 | 33.7 ± 4.6 | .68 |
|
| |||
| Distance of STJ/LVOT (mm) | 20.1 ± 10.5 | 19.4 ±3.2 | .62 |
|
| |||
| Aortic root calcification (%) | |||
|
| |||
| Annular calcification | .49 | ||
|
| |||
| Mild | 66.7 | 71.2 | |
|
| |||
| Moderate | 27.5 | 27.1 | |
|
| |||
| Severe | 5.9 | 1.7 | |
|
| |||
| Sinotubular calcification | 5.9 | 8.5 | .44 |
|
| |||
| LVOT calcification | 19.6 | 11.9 | .19 |
|
| |||
| Mitral annular calcification | 15.7 | 18.6 | .44 |
|
| |||
| LVOT/AAo angle (°) | 13.7 ± 5.1 | 13.9 ± 5.2 | .84 |
|
| |||
| AAo angle (°) | 46.5 ± 9.4 | 47.5 ± 12.1 | .62 |
AAo, ascending aorta; Ao, aorta; EF, ejection fraction; HFHG, high flow-high gradient; LFLG, low flow-low gradient; LVOT, left ventricular outflow tract; PG, pressure gradient; STJ, sinotubular junction; TEE, transesophageal echocardiography.
Procedural data in relation to the type of valve used
There were few differences in procedural data related to valve design and sheath size as shown on table 3.
Table 3. Procedural data associated with each type of valve
| Type of valve | P | ||
|---|---|---|---|
|
| |||
| Evolut PRO (N = 51) | SAPIEN 3 (N = 59) | ||
| Route (%) | .51 | ||
|
| |||
| Right femoral | 60.8 | 59.3 | |
|
| |||
| Center femoral | 39.2 | 40.7 | |
|
| |||
| Annulus by TEE (mm) | 23.8 ± 2.1 | 24.5 ± 1.9 | .07 |
|
| |||
| Balloon size (mm) | 22.5 ± 1.9 | 22.6 ± 1.9 | .63 |
|
| |||
| Balloon sizing (mm) | 23.4 ± 1.7 | 23.6 ± 1.9 | .44 |
|
| |||
| Valve size (%) | |||
|
| |||
| 23 | 0.0 | 30.5 | |
|
| |||
| 26 | 43.1 | 45.8 | |
|
| |||
| 29 | 56.9 | 23.7 | |
|
| |||
| Sheath size (Fr) | 16.0 | 14.5 ± 0.9 | < .001 |
|
| |||
| Sheath outer diameter (mm) | 7.3 ± 0.1 | 6.2 ± 0.3 | < .001 |
|
| |||
| Femoral artery diameter (mm) | 7.9 ±1.1 | 8.2 ± 0.9 | .20 |
|
| |||
| Sheath femoral artery ratio | 0.9 ± 0.1 | 0.8 ± 0.1 | < .001 |
|
| |||
| Cover index (%) | |||
|
| |||
| TEE | 16.4 ± 5.6 | 5.2 ± 4.2 | < .001 |
|
| |||
| Balloon | 18.3± 3.3 | 8.9 ± 3.3 | < .001 |
|
| |||
| Valve mean pressure gradient | 9.8 | 12.2 | .01 |
|
| |||
| AR index (%) | 28.4 ± 7.8 | 30.7 ± 7.4 | .11 |
|
| |||
| Implantation depth (mm) | |||
|
| |||
| LCC | 5.8 ± 2.3 | 4.2 ± 1.7 | < .001 |
|
| |||
| NCC | 6.3 ± 2.5 | 5.27 ± 1.7 | .01 |
|
| |||
| Amount of contrast (mL) | 145.5 ± 48.8 | 128.6 ± 33.2 | .03 |
|
| |||
| Radiation (mGy) | 4944.4 ± 2294.8 | 4557.8 ± 3133.9 | .46 |
AR, aortic regurgitation; LCC, left coronary cusp; NCC, non-coronary cusp; TEE, transesophageal echocardiography.
Outcomes in association with the type of valve used
There was a significant difference in PVL (both immediate and at hospital discharge) and consequently more balloon postdilatation in the Evolute compared to the SAPIEN 3 group. The use of significantly larger amounts of contrast with the Evolut PRO valves may explain the increased number of acute kidney injury described in this group compared to the SAPIEN valve group. Results were favorable to the SAPIEN 3 valve regarding the endpoints of stroke or in-hospital mortality. However, no statistically significant differences were reported. The rates of device success (absence of a significant PVL (≥ grade II) at hospital discharge, need for second valve implantation, valve embolization, the performance of the prosthetic heart valve, and mortality) were 86% and 98% with the Evolut PRO and SAPIEN 3 valves, respectively; P = .01 (table 4).
Table 4. In-hospital outcomes in patients treated with the Evolut PRO vs the SAPIEN 3 valve
| Type of valve | P | ||
|---|---|---|---|
|
| |||
| Evolut PRO (N = 51) | SAPIEN 3 (N = 59) | ||
| Immediate PVL | .01 | ||
|
| |||
| No/trace | 19 (37.3) | 46 (78) | |
|
| |||
| Grade I | 22 (43.1) | 9 (15.2) | |
|
| |||
| ≥ grade II | 10 (19.6) | 4 (6.8) | |
|
| |||
| Balloon postdilatation | 8 (15.7) | 3 (5.1) | .35 |
|
| |||
| PVL at discharge | .01 | ||
|
| |||
| No/trace | 26 (50.9) | 49 (83.1) | |
|
| |||
| Grade I | 22 (43.1) | 9 (15.3) | |
|
| |||
| Grade II | 2 (3.9) | 1 (1.7) | |
|
| |||
| Grade III | 1 (2) | 0 | |
|
| |||
| Grade IV | 0 | 0 | |
|
| |||
| Overall new-onset conduction defects | 9 (17.6) | 10 (16.9) | .56 |
|
| |||
| New-onset LBBB | 4 (7.8) | 4 (6.7) | .40 |
|
| |||
| Postoperative pacemaker implantation | 4 (7.8) | 3 (5.1) | .25 |
|
| |||
| Vascular complications | .66 | ||
|
| |||
| Major vascular complications | 2 (3.9) | 2 (3.4) | |
|
| |||
| Minor vascular complications | 4 (7.9) | 3 (5.1) | |
|
| |||
| Bleeding complications | 0 | 0 | |
|
| |||
| Acute kidney injury* | 3 (5.9) | 2 (3.4) | .28 |
|
| |||
| Stroke | 1 (2) | 0 | .46 |
|
| |||
| Valve embolization | 1 (2) | 0 | .46 |
|
| |||
| Need for second valve | 2 (3.9) | 0 | .30 |
|
| |||
| In-hospital mortality rate | 2 (3.9) | 0 | .30 |
Data are expressed as no. (%). PVL, paravalvular leak.
*Acute kidney injury including all stages of the disease.
Impact of anatomical factors on PVL
Calcification and the LVOT/AAo angle had a greater impact on PVL in the Evolut PRO compared to the SAPIEN 3 valve. The LVOT/AAo angle was categorized based on the receiver operating characteristic (ROC)-derived cut-off value for the endpoint of significant PVL ≥ grade II: cut-off value = 11º, 80% sensitivity, and 35.8% specificity, area under the curve (0.57; 95% confidence interval, 0.474-0.666; P = .37.) On the other hand, the AA angle did not seem to be very relevant to PVL within the groups (table 5).
Table 5. Association between anatomical factors and PVL in patients treated with the Evolut PRO vs the SAPIEN 3 valves
| Evolut PRO valve (N = 51) | SAPIEN 3 Valve (N = 59) | pa | pb | pc | |||
|---|---|---|---|---|---|---|---|
|
| |||||||
| < Mild PVL | ≥ Mild PVL | < Mild PVL | ≥ Mild PVL | ||||
| Number | 37.3 | 62.7 | 77.9 | 22.0 | .01 | ||
|
| |||||||
| Annular calcification | .03 | .2 | |||||
|
| |||||||
| Mild | 31.4 | 35.3 | 59.3 | 11.9 | .001 | ||
|
| |||||||
| Moderate | 5.9 | 21.6 | 16.9 | 10.2 | .024 | ||
|
| |||||||
| Severe | 0.0 | 5.9 | 1.7 | 0.0 | .046 | ||
|
| |||||||
| LVOT calcification | 1.7 | 17.6 | 3.4 | 8.5 | .04 | .001 | .323 |
|
| |||||||
| Mitral annular calcification | 0.0 | 15.7 | 15.3 | 3.4 | .001 | 0.2 | .035 |
|
| |||||||
| LVOT/AAo anglea | .01 | .001 | |||||
|
| |||||||
| < 11° | 17.6 | 15.7 | 25.4 | 1.7 | .002 | ||
|
| |||||||
| ≥ 11° | 19.6 | 47.1 | 52.5 | 20.3 | .03 | ||
|
| |||||||
| AAo angle (%) | .78 | .34 | |||||
|
| |||||||
| < 48° | 23.5 | 37.2 | 45.8 | 15.2 | .003 | ||
|
| |||||||
| > 48° | 13.7 | 25.5 | 32.2 | 6.8 | .001 | ||
LVOT, left ventricular outflow tract; AAo, ascending aorta. An LVOT/AAo angle of 11º is the cut-off vale for the rate of PVL as detected by the ROC curve. Data are expressed as percentage (%).
aP value within the Evolut PRO group.
bP value within the SAPIEN 3 group.
cP value from a chi-square score between both groups.
Table 6 shows the univariate analysis of predictors of ≥ grade II PVL immediately after the procedure. As demonstrated, moderate and severe valvular calcification, LVOT calcification, and the LVOT/AAo angle contribute to PVL significantly.
Table 6. Univariate analysis of predictors of significant immediate postoperative PVL (grade ≥ 2)
| Variable | Univariate | |
|---|---|---|
|
| ||
| OR (95%CI) | P | |
| Severe calcification | 35.000 (3.138-390.431) | .004 |
|
| ||
| LVOT calcification | 10.921 (3.208-37.174) | < .001 |
|
| ||
| LVOT/AAo angle | 1.047 (0.940-1.165) | .003 |
|
| ||
| AA | 1.016 (0.967-1.067) | .524 |
|
| ||
| Valve type (Evolut PRO) | 2.750 (0.872-8.669) | .084 |
|
| ||
| TEE cover index | 1.099 (1.018-1.188) | .016 |
|
| ||
| Cover index by balloon sizing | 1.108 (1.001-1.226) | .049 |
|
| ||
| LCC implantation depth | 1.199 (0.953-1.510) | .122 |
|
| ||
| RCC implantation depth | 1.167 (0.914-1.489) | .215 |
P value was significant if < .05. 95%IC, 95% confidence interval; AA, aortic angulation; AAo, ascending aorta; AR, aortic regurgitation; LCC, left coronary cusp; LVOT, left ventricular outflow tract; PVL, paravalvular leak; RCC, right coronary cusp; TEE, transesophageal echocardiography.
Impact of LVOT/AAo and AA angles on implantation depth
There was a significant negative correlation between the implantation depth of the Evolut PRO valve at the NCC and LVOT/AAo angles (r = -0.38; P = .01). There was no such correlation with the SAPIEN 3 valve (table 7).
Table 7. Correlation of implantation depth (in both valves) with the LVOT/AAo and AA angles
| Type of valve | ||||
|---|---|---|---|---|
|
| ||||
| Evolut PRO | SAPIEN 3 | |||
|
| ||||
| LCC | NCC | LCC | NCC | |
| LVOT/AAo angle (°) | -0.23 (0.09) | -0.38 (0.01) | 0.09 (0.46) | 0.16 (0.21) |
|
| ||||
| AAo angle (°) | 0.13 (0.33) | 0.06 (0.65) | 0.02 (0.87) | 0.06 (0.61) |
r indicates strength of correlation and P value indicates significance of correlation. P value was significant if < .05. AAo, ascending aorta; LCC, left coronary cusp; LVOT, left ventricular outflow tract; NCC, non-coronary cusp.
DISCUSSION
In this study 2 important findings were made. First, implantation of the Evolut PRO valve was associated with a higher risk of significant PVL compared to the SAPIEN 3 valve. Secondly, the rate of PPI was equal in both groups. Otherwise, both types of valves yielded similar outcomes.
Reducing PVL is an important challenge regarding TAVI as it is associated with worse outcomes especially with the current use of these devices in lower-risk patients.14
A randomized comparison between the CoreValve and SAPIEN XT valves in the CHOICE trial revealed a lower rate of moderate-to-severe PVL in the SAPIEN XT group.15 In the SOLVE-TAVI trial, the non-inferiority of 2 devices (SAPIEN 3 and Evolut R) was reported in terms of their primary efficacy composite endpoint (death, stroke, paravalvular regurgitation, and new pacemaker implantation).16 Currently, the SAPIEN 3 Ultra and Evolut PRO+ have been developed with early favorable outcomes.17
In our study, relevant PVL (≥ grade II) was more common in patients who received the Evolut PRO compared to the SAPIEN 3 valve (9.6% vs 6.8%, respectively). Enríquez-Rodríguez et al. reported a lower rate (2.5%) of moderate to severe PVL with the SAPIEN 3 valves possibly due to the presence of an external sealing cuff.18
Obviously, anatomical factors are important for the occurrence of PVL. We observed that larger LVOT/AAo angles were associated with a higher rate of PVL, particularly with the Evolut PRO valve. Sherif et al. demonstrated that the risk of PVL increases with larger LVOT/AAo angles.10 We also observed that the LVOT/AAo angle affects implantation depth in association with the NCC with the Evolut PRO, but not with the SAPIEN 3 valves. It is quite conceivable that implantation depth impacts the rate of PVL.
Sherif et al. were the first ones to report on the association between increased AA angles and postoperative PVL with self-expanding valves.10 A subsequent retrospective study conducted by Abramowitz et al. described a higher rate of complications (eg, postoperative PVL in patients with horizontal aortas (defined by an AA ≥ 48º as seen on the cardiac CT scan) who received self-expanding valves.11 We observed that AA angles impacted PVL in patients who received Evolut PRO valves even if these angles were < 48º with no significant differences in the rate of PVL for AA angles < 48º or ≥ 48º.
In this study we also observed 6 patients with AA angles ≤ 30º (3 patients with Evolut PRO and 3 patients with SAPIEN 3). All of them were free of PVL immediately after valve deployment. One could speculate that AA angles ≤ 30º are the best for Evolut PRO valve implantation, but the small size of the sample prevents us from drawing any definitive conclusions.
In our study, the rates of device success determined by the absence of a significant PVL (≥ grade II) at hospital discharge, need for a second valve, valve embolization, the performance of the prosthetic heart valve, and the mortality rate according to VARC definition9 were 86% and 98% with the Evolut PRO and SAPIEN 3 valve, respectively. Similarly, Li et. al found a high device success rate for both the SAPIEN 3 and the Evolut R valve (94% and 96%, respectively).19
We found similar rates of postoperative conduction defects and PPI for both Evolut PRO and SAPIEN 3 valve types (7.8% and 5.1%, respectively). Popma et al.20 and Vlastra et al.21 reported lower rates of PPI with new generation balloon expandable valves compared to new-generation self-expanding valves. The comparable rates of conduction defects and PPI with either valve in our study was probably due to the lower implantation depth of Evolut PRO valves.
Li et al. reported higher rates of postdilatation of up to 30% with the Evolut R compared to the SAPIEN 3 valve.19 This was not seen in our study (15.7% and 5.1%, respectively; P = .35). This was probably so thanks to the proper positioning of the Evolut PRO valve and routine predilatation in all our cases.
In this study, in-hospital mortality was similar in both valve groups. Li et al. also reported that mortality was not associated with the type of valve implanted.19 The CHOICE trial also showed a comparable mortality rate with the use of older-generation valves (Core- Valve and SAPIEN XT).15
The rates of stroke were similar for both the Evolut PRO and the SAPIEN 3 valve and lower compared to those seen with older generation devices.15,19,22,23 The operators' experience and improved delivery systems are likely to account for the reduced risk of thromboembolic complications.
Regardless of the type of valve used, acute kidney injury seemed to be slightly more common in our study (5.9% and 3.5% for the Evolut PRO and the SAPIEN 3, respectively) than previously reported. Husser et al.24 noted a rate of 2.7% in SAPIEN 3 valves while Kodali et al.25 reported rates of 1.7%. However, large multicenter studies usually have stricter inclusion criteria so the baseline kidney function of the patients included was better.19
Despite increased sheath/femoral artery ratios with the Evolut PRO valve, the rate of bleeding or vascular complications was similar compared to the SAPIEN 3 valve. Similar results were reported by Li et al.19 and Panchal et al.26
Limitations
This was a single-center study with a small sample size and limited statistical power. As routine computed tomography scan was not part of our study, specific information on the anatomy of the aortic root was not available and no adjustment was performed based on the annular dimensions or degree/distribution of aortic annular calcification. Also, angiography-based measurements of the LVOT/AAo and AA angles may be inaccurate. However, this may have helped exclude selection bias as some operators are reluctant to use self-expanding valves in view of heavy calcifications or severe angulation.
Follow-up was limited to the length of stay (average 1 week). However, this seems reasonable since we focused on procedural aspects. Furthermore, in comparable studies, in-hospital outcome and 30-day follow-up results were quite similar.
CONCLUSIONS
This randomized study demonstrated comparable procedural and in-hospital outcomes for the Evolut PRO and SAPIEN 3 valves except for a significantly higher rate of PVL associated with the Evolut PRO valves. The PVL reported was associated with the LVOT/AAo angle in Evolut PRO group, which also impacted negatively the implantation depth of this type of valve.
AUTHORS' CONTRIBUTIONS
Idea and design: H. M. Elnaggar, M. S. Mahmoud, W. Schoels, and Y. T. Kishk. Administrative support: W. Schoels, M. Kullmer, and M. Dia. Provision of study materials or patients: M. S. Mahmoud, M. Algowhary, and H. M. Elnaggar. Data collection and assembly: M. S. Mahmoud, M. Kullmer, and M. Dia. Data analysis and interpretation: M. S. Mahmoud, Y. T. Kishk, M. Algowhary, and H. M. Elnaggar. Manuscript drafting and final approval: all authors.
WHAT IS KNOWN ABOUT THE TOPIC?
Self-expanding (Evolut platform) and balloon-expandable (SAPIEN series) valves are the most commonly used TAVI devices.
Outcomes between both types of valves are similar with a relative increase of PVL and conduction defects in the Evolut type.
Also, there are some anatomical challenges when deploying self-expanding valves such as severe aortic angulation (horizontal aorta).
There is no prospective randomized clinical trials comparing Evolut PRO (self-expanding valve with external skirt) to SAPIEN 3 valves.
WHAT DOES THIS STUDY ADD?
This is considered the first prospective randomized clinical trial that compared the Evolut PRO valve (self-expanding valve with external skirt) to the SAPIEN 3 valve.
This study demonstrated comparable favorable outcomes between both types of valves apart from a significantly higher PVL in the Evolut PRO group.
Also, in our study, LVOT/AAo and AA angulation had an impact on PVL in the Evolut PRO group compared to the SAPIEN 3 group. However, AA angulation had no impact on PVL within the groups.
The LVOT/AAo angle was negatively associated with implantation depth in the case of the Evolut PRO valve with no effect on SAPIEN 3 valves whatsoever, which may have impacted the development of PVL in the Evolut PRO group.
REFERENCES
1. Athappan G, Patvardhan E, Tuzcu EM, et al. Incidence, predictors, and outcomes of aortic regurgitation after transcatheter aortic valve replacement:meta-analysis and systematic review of literature. J Am Coll Cardiol. 2013;61:1585-1595. [ Links ]
2. Herrmann HC, Abdel-Wahab M, Attizzani GF, et al, Rationale and design of the SMall Annuli Randomized to Evolut or SAPIEN Trial (SMART Trial). Am Heart J. 2022;243:92-102. [ Links ]
3. Paitazoglou C, Meincke F, Thorsten Hanke M, et al. The ALSTER-TAVI All-Comers Registry:Procedural and 1-Year Clinical Outcomes of Balloon-Expandable vs Self-Expanding Contemporary TAVI Valves. J Invasive Cardiol. 2021;33:E356-E364. [ Links ]
4. Mahmoud MS, Kishk YT, Algowhary M, et al. Balloon Sizing for Transcatheter Aortic Valve Implantation Using 3 rd Generation Valves, Does It Still Work?Int Med J. 2021;28:604-609. [ Links ]
5. Détaint D, Lepage L, Himbert D, et al. Determinants of significant paravalvular regurgitation after transcatheter aortic valve implantation:impact of device and annulus discongruence. JACC Cardiovasc Interv. 2009;2:821-827. [ Links ]
6. Wang J, Yu W, Jin Q, et al. Risk factors for post-TAVI bleeding according to the VARC-2 bleeding definition and effect of the bleeding on short-term mortality:a meta-analysis. Can J Cardiol. 2017;33:525-534. [ Links ]
7. Sellers RD, Levy MJ, Amplatz K, Lillehei CW. Left retrograde cardioangiography in acquired cardiac disease:Technic, indications and interpretations in 700 cases. Am J Cardiol. 1964;14:437-447. [ Links ]
8. Sinning JM, Hammerstingl C, Vasa-Nicotera M, et al. Aortic regurgitation index defines severity of peri-prosthetic regurgitation and predicts outcome in patients after transcatheter aortic valve implantation. J Am Col Cardiol. 2012;59:1134-1141. [ Links ]
9. Kappetein AP, Head SJ, Généreux P, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation:the Valve Academic Research Consortium-2 consensus document. J Thorac Cardiovasc Surg. 2013;145:6-23. [ Links ]
10. Sherif MA, Abdel-Wahab M, Stöcker B, et al. Anatomic and procedural predictors of paravalvular aortic regurgitation after implantation of the Medtronic CoreValve bioprosthesis. J Am Col Cardiol. 2010;56:1623-1629. [ Links ]
11. Abramowitz Y, Maeno Y, Chakravarty T, et al., Aortic angulation attenuates procedural success following self-expandable but not balloon-expandable TAVR. JACC Cardiovasc Imaging. 2016;9:964-972. [ Links ]
12. Di Stefano D, Colombo A, Mangieri A, et al. Impact of horizontal aorta on procedural and clinical outcomes in second-generation transcatheter aortic valve implantation. EuroIntervention. 2019;15:e749-e756. [ Links ]
13. Mostafa AE, Richardt G, and Abdel-Wahab M. Clinical utility of a predictive model for paravalvular aortic regurgitation after transcatheter aortic valve implantation with a self-expandable prosthesis. Egypt Heart J. 2017;69:253-259. [ Links ]
14. Reardon MJ, Van Mieghem NM, Popma JJ, et al. Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2017;376:1321-1331. [ Links ]
15. Abdel-Wahab M, Mehilli J, Frerker C, et al. Comparison of balloon-expandable vs self-expandable valves in patients undergoing transcatheter aortic valve replacement:the CHOICE randomized clinical trial. JAMA Cardiol. 2014;311:1503-1514. [ Links ]
16. Webb J, Wood D, Sathananthan J, Landes U. Balloon-expandable or self-expandable transcatheter heart valves. Which are best?Eur Heart J. 2020;41:1900-1902. [ Links ]
17. Jiang T, Hasan SM, Faluk M, Patel J. Evolution of Transcatheter Aortic Valve Replacement|Review of Literature. Curr Probl Cardiol. 2021;46:100600. [ Links ]
18. Enríquez-Rodríguez E, Amat-Santos IJ Jiménez-Quevedo P, et al. Comparison of the hemodynamic performance of the balloon-expandable SAPIEN 3 versus self-expandable Evolut R transcatheter valve:a case-matched study. Rev Esp Cardiol. 2018;71:735-742. [ Links ]
19. Li Y-M, Tsauo J-Y, Liao Y-B, Zhao Z-G, Chen M. Comparison of Third Generation Balloon-Expandable Edwards Sapien 3 Versus Self-Expandable Evolut R in Transcatheter Aortic Valve Implantation:A Meta-Analysis. Ann Palliat Med. 2020;9:700-708. [ Links ]
20. Popma JJ, Reardon MJ, Khabbaz K, et al. Early clinical outcomes after transcatheter aortic valve replacement using a novel self-expanding bioprosthesis in patients with severe aortic stenosis who are suboptimal for surgery:results of the Evolut R US study. JACC Cardiovasc Interv. 2017;10:268-275. [ Links ]
21. Vlastra W, Chandrasekhar J, Muñoz-Garcia AJ, et al. Comparison of balloon-expandable vs. self-expandable valves in patients undergoing transfemoral transcatheter aortic valve implantation:from the CENTER-collaboration. Eur Heart J. 2019;40:456-465. [ Links ]
22. Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364:2187-2198. [ Links ]
23. Adams DH, Popma JJ, Reardon MJ, et al. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med. 2014;370:1790-1798. [ Links ]
24. Husser O, W.-K. Kim, et al. Multicenter comparison of novel self-expanding versus balloon-expandable transcatheter heart valves. JACC Cardiovasc Interv. 2017;10:2078-2087. [ Links ]
25. Kodali S, Thourani VH, White J, et al. Early clinical and echocardiographic outcomes after SAPIEN 3 transcatheter aortic valve replacement in inoperable, high-risk and intermediate-risk patients with aortic stenosis. Eur Heart J. 2016;37:2252-2262. [ Links ]
26. Panchal HB, Barry N, Bhatheja S, Albalbissi K, Mukherjee D, Paul T. Mortality and major adverse cardiovascular events after transcatheter aortic valve replacement using Edwards valve versus CoreValve:A meta-analysis. Cardiovasc Revasc Med. 2016;17:24-33. [ Links ]
Received: July 07, 2022; Accepted: October 24, 2022











 texto em
texto em 


