INTRODUCTION
Advancement in the field of nanotechnology have resulted in “Nano-medicine.” Robert A. Freitas Jr. described this concept first in the year 1993 which included treatment of human biological system by using nanostructures or particle at the molecular level [1]. Nanomaterials are widely explored in Periodontics, as well as controlled drug release in forms of hollow spheres, core-shell, nanotubes and other active components.
Periodontal disease is an inflammatory and infectious disease of gingiva that leads to the breakdown of periodontal tissues. Its severe form results in loss of alveolar bone with eventual loss of the tooth. The current evidence on etiopathogenesis suggests that the major risk factors are the bacterial aggression and the host response influenced by behavioral and systemic risk factors [2].
Microbial etiology is treated by administering antimicrobials as an adjunct to Scaling & Root Planing (SRP). This enhances the treatment outcome and can also counter the shortcomings in usage of systemic antimicrobials [3]. These antimicrobials are used primarily in the form of oral rinses, irrigation or by topical application. But these modalities do not reach subgingival areas predictably. So a better option would be subgingival delivery of the drug in the periodontal pocket which cannot be washed off like in case of topical application [4]. Treatment with antibiotic as local drug delivery may be necessary, especially in the treatment of localized disease [5].
Tetracycline is usually considered a drug of choice, because of its wide spectrum of activity against periodontal pathogen [6], its potential to concentrate in gingival crevicular fluid (GCF) within the periodontal sulcus [7], its anticollagenase effect [8] and bacteriostatic range for most putative periopathogens [9]. Local and intracrevicular antimicrobial therapy is consistent with the site-specific nature of disease and allows for controlled sustained release while minimizing systemic exposure. Tetracycline HCl possess the ability to bind to the hard tissue walls of the pockets and thus establish a drug reservoir following subgingival irrigation with concentrated solutions [10].
Recent advances in nanotechnology have introduced new therapeutic materials for periodontal diseases. Nanoparticles are clusters of atoms in the size range of 1-100 nm [11]. Silver nanoparticles have the ability to anchor to the bacterial cell wall and subsequently penetrate it, thereby causing structural changes in cell wall affecting its permeability and hence, death of the cell. The antimicrobial efficacy of 0.02mg/gm silver nanoparticles gel against P. gingivalis is 3.125μg/ml and 6.25μg/ml for A. actinomycetemcomitans making it suitable option as nano-medicine [12]. The present study evaluates the efficacy of the silver nanoparticles in comparison with Tetracycline in chronic periodontitis patients.
METHODS
SCREENING AND SELECTION OF THE SUBJECTS
The subjects screened and diagnosed of chronic periodontitis were included for this clinical trial. 60 sites in patients were selected, being divided into three groups randomly (coin test method). An informed consent from these subjects was obtained before the clinical trial.
INCLUSION AND EXCLUSION CRITERIA
Systemically healthy subjects with probing pocket depth of 4-6 mm from age group of 30-50 years were included.
Pregnant, lactating women, allergic to tetracycline and/or silver nanoparticles and the patients with history of any previous periodontal or antibiotic therapy were excluded.
ALLOCATION TO THE GROUP
60 sites meeting the inclusion criteria were randomized into three treatment groups 20 sites each:
Group A: Scaling and root planing followed by subgingival delivery of silver nanoparticles gel
Group B: Scaling and root planing followed by subgingival delivery of tetracycline gel
Group C: Only scaling and root planing
INDIGENOUS GEL PREPARATION
The materials used in this study were 0.02% Silver nanoparticles gel and 5% Tetracycline gel indigenously prepared in the pharmacy department of our institute. An injectable system was used for drug delivery into the periodontal pocket.
PREPARATION OF 0.02% SILVER NANOPARTICLES GEL [12]
Hydroxypropyl methylcellulose (HPMC) powder [13] and silver nanoparticles powder were used to prepare the silver nanoparticle gel. HPMC powder is believed to have several desirable properties for gel formation. It is non-irritating, high in viscosity with increased stability, negligible batch-to-batch variability, unaffected by aging and avoids bacterial or fungal growth [14]. For every 10g of gel, 10 ml of distilled water and HPMC powder was used and this formulation was stirred very well. To this 20 µg of silver nanoparticle was added and with the help of automated sonicator, the gel for obtained in 2 hours of continuous stirring.
PREPARATION OF 5% TETRACYCLINE GEL [15]
The tetracycline gel was prepared using HPMC powder and tetracycline HCl powder. To prepare 10gms of gel, 10ml of distilled water was taken and HPMC powder was added while stirring. To this 500 µg tetracycline HCl powder was added with continuous stirring for 2 hours using automated sonicator to make tetracycline gel.
RECORDING OF CLINICAL PARAMETERS
All the clinical and microbiological measurements were performed by a single calibrated examiner. University of North Carolina Probe (UNC-15) was used to measure the Probing Pocket Depth and Clinical Attachment Level to the nearest millimeter. Acrylic occlusal stents standardized the positioning and insertion plane for the probe. The clinical parameters recorded at baseline, 1 month and 3 months were Silness & Loe’s Plaque Index (PI), Loe and Silness’s Gingival Index (GI), Probing Pocket Depth (PPD) and Clinical Attachment Level (CAL).
PROCEDURE
Supragingival and subgingival scaling and root planing was performed at initial visit and the patients were kept on follow-up. On the following appointment, only patients with persistent periodontal pockets (PPD of 4-6 mm) were selected. In the same appointment, study sites were determined and alginate impression were made for fabricating an acrylic stent, subsequently acrylic occlusal stents were fabricated for standardization of clinical recordings during the entire study period.
Patients were recalled after 1 month, acrylic stents were placed over the test site and baseline measurements were recorded using a UNC-15 periodontal probe. The experimental drugs were delivered subgingivally in the selected sites using syringe and blunt cannula till the pockets were overfilled with the gel. The experimental sites were then covered with Coe-Pak to retain the material in the periodontal pockets, as well as to prevent the ingression of oral fluids. Subjects were recalled after 7 days for removal of the periodontal dressing. For Group C, only SRP was done and the recall pattern was similar to Group A and B. Re-assessment of the clinical parameters was done at 1 and 3 months. Between these appointments, the patients were encouraged to maintain the oral hygiene. The patients were refrained from using any other chemical plaque control measures during the trial period. Patients were asked to report immediately on developing any untoward reaction. Expected adverse effects were hypersensitivity and local reaction.
MICROBIOLOGICAL ASSESSMENT
At baseline, the study sites were isolated with the help of cotton rolls. The subgingival plaque samples were collected with sterile curette and transferred into 5 ml of thioglycollate broth. Thioglycollate medium (0.1 ml) was used with the blood agar plate due to its recommendation for the cultivation of aerobic, microaerophilic and anaerobic microorganism. Blood agar consists of a base containing a protein source (e.g. Tryptones), soya bean protein digest, sodium chloride, agar and 5% sheep blood. L shape spreader was used to spread the sample evenly on the blood agar. The plates were placed into anaerobic gas pack. Anaerobic gas pack contains an oxygen absorbing and carbon dioxide generating agent. It also contains methylene blue as an indicator which assures maintenance of anaerobic condition of the gas pack. The gas pack was sealed carefully as oxygen absorption and carbon dioxide generation starts immediately on contact with air. Subsequently, the pack was placed into the incubator for 72 hours at 37 °C. After the incubation period the agar plates were assessed for the total number of colony forming units of anaerobic bacteria. The plate was divided into grids to aid counting. The total colony forming units were then attained by summing up the grids.
After 3 months, the above procedure was repeated.
Data was subjected to statistical analysis using Statistical package for social sciences (SPSS v 21.0, IBM). Normality of numerical data (all indices) was checked using Shapiro-Wilk test & was found that the data followed a normal curve; hence parametric tests have been used for comparisons. Inter group comparison was done using “one-way ANOVA” followed by pair wise comparison using “post- hoc tukey test”. Intra group comparison was done using “paired t-test”. Comparison of frequencies of categories of variables with groups was done using chi- square test.
RESULTS
All patients showed good compliance. The healing period was uneventful for all the treated groups, without any signs of inflammation, swelling and allergy indicating the biocompatibility of the materials.
Table 1 shows intragroup comparison between all the clinical parameters recorded and compared. P value of p<0.0001 was achieved in all the groups i.e. at every step the p value was found to be less than 0.05 whic is statistically significant in Plaque Index, Gingival Index Probing Pocket Depth, CAL and Colony Forming Unit.
Table 1. Intra-group comparison of clinical parameters
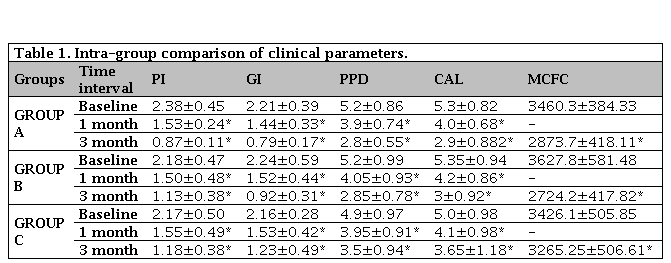
PI: Plaque Index; GI: Gingival Index; PPD: Probing Pocket Depth; CAL: Clinical Attachment Level; MCFC: Mean Colony Forming Cells. * p<0.0001 statistically significance from baseline to one month, baseline to three months and from one month to three months. Values are expressed as mean±standard deviation.
Plaque index showed reduction in values in all the three groups. The reduction of mean value from 2.38 ± 0.45 to 0.87 ± 0.11 for tetracycline group, silver nanoparticle showed improvement to 1.13 ± 0.38 from 2.18 ± 0.47 whereas SRP group reduced from 2.17±0.50 to 1.18±0.38. Similarly, gingival index assessing the tetracycline group and silver nanoparticle showed considerable improvement in gingival status from 2.21 ± 0.39 to 0.79 ± 0.17 and 2.24 ± 0.59 to 0.92 ± 0.31 respectively. The SRP group C proved to be little less effective in comparison to tetracycline and silver nanoparticle group with baseline value of 2.16 ± 0.28 reducing to 1.23 ± 0.49 in 3 months.
The baseline value of PPD ranged from 4.9-5.2. Group A and B showed reduction to 2.8 ± 0.55 and 2.85 ± 0.78, respectively, whereas Group C without local drug delivered showed reduction of 3.5 ± 0.94 only (Figure 1). Likewise, the clinical attachment gain was comparatively poor with Group C. The colony forming unit values of all three groups showed very poor reduction in Group C from 3426.1 ± 505.85 to 3265.25 ± 506.61. On contrary Group A and Group B showed reduction from 3460.3 ± 384.33 to 2873 ± 418.11 and 3627.8 ± 581.48 to 2724.5 ± 417.82 respectively (Figure 2).
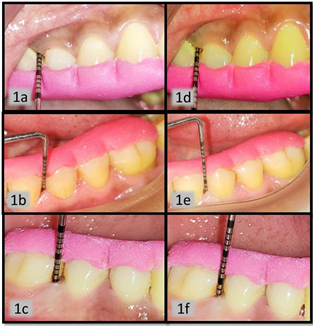
Figure 1. Figure 1a, b, c are baseline probing pocket depth clinical picture of group A, B, C respectively. The baseline evaluation was recorded in range 5-6mm of PPD. Figure 1d, e, f are postoperative PPD recorded 3 month post-operatively for group A, B and C, respectively, which was found to be in range of 3-4 mm.PPD: Probing Pocket Depth.
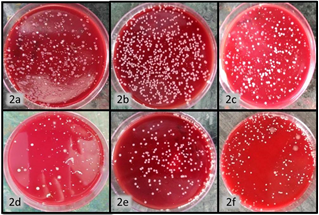
Figure. Figure 2a, b, c are baseline CFU A, B, C respectively. Figure 2d, e, f are postoperative CFU evaluated 3 month post-operatively for group A, B, C respectively.CFU: Colony forming units.
Intergroup comparison can be appreciated in Table 2 between the clinical parameters. Group A and group B showed no statistical significant difference throughout the study period. When group C was compared with Group A and group B statistical significant difference were obtained from baseline to 3 months. Whereas baseline to one month showed no significant results.
Table 2. Inter-group comparison of clinical parameters

PI: Plaque Index; GI: Gingival Index; PPD: Probing Pocket Depth; CAL: Clinical Attachment Level; MCFC: Mean Colony Forming Cells. * p<0.05. Values are expressed as mean±standard deviation.
The result values clearly states that only scaling and root planning did not yield much result. When this group was compared with local drug delivery group, statistical significant results were obtained. Group A and group B showed no statistical significant result meaning either of the drugs (tetracycline or silver nano-particles) can be used as local drug delivery agent.
DISCUSSION
The present randomized clinical trial was carried out with the objective to assess the effectiveness of silver nanoparticles gel and tetracycline gel as local drug delivery systems. Along with SRP, clinical and microbiological outcomes of non-surgical periodontal therapy in the chronic periodontitis patients were assessed. All the clinical assessments were performed using UNC 15 periodontal probe with the visual read out. Mayfield et al. [16] observed better intra and inter individual reproducibility using a manual probe in comparison to simple TPS and automated force-controlled probes (Florida probe).
In Group A, there was statistically significant reduction in mean PI from baseline to 3 months (p<0.05). This can be correlated to Masurkar SA et al. [17] who concluded that, silver nanoparticles can enhance quorum quenching activity against Staphylococcus aureus and prevent biofilm formation. The mean gingival index showed statistically significant (p<0.05) reduction in Group A from baseline to 3 months. This can be correlated to Prasetyo BC et al. [18] who concluded that Coe-Pak dressings with the addition of silver nanoparticles tended to give better results in tissue healing due to anti-inflammatory and antimicrobial properties of silver nanoparticles. Similar, anti-inflammatory and antimicrobial effects of silver nanoparticles were demonstrated by Habiboallah G et al. [19].
In Group A the mean PPD significantly reduced from 5.20 ± 0.86 to 2.80 ± 0.55 baseline to 3 months (p<0.05). This can be correlated with antibacterial effect of silver nanoparticles by elimination or reduction of pathologic microbial flora. This was in accordance to Tian X et al. [20] who suggested that silver nanoparticles being smaller in size can penetrate into the deeper layers of biofilm and ultimately leads to the destruction of microbial cells. In Group B also, mean PPD was reduced significantly from 5.20 ± 0.99 to 2.85 ± 0.78 from baseline to 3 months, which was statistically significant (p<0.05). This was in accordance with previous study done by Jeongin S N et al. [21] who found significant reduction in probing pocket depth when tetracycline was used as an adjunct to SRP.
To assess the CAL, the pair-wise comparison between various follow-up levels within Group A was performed. It was observed that, there was improvement in clinical attachment level from baseline to 3 months, which was statistically significant (p<0.05). This can be attributed to bactericidal, anti-inflammatory and antimicrobial properties of silver nanoparticles. Sadeghi et al. [22] concluded that silver nanoparticles shows, bactericidal effect against S. mutans. Similar, properties were demonstrated by Monteiro et al. [23]. These results for CAL were in contrast to the study done by Bonito AJ et al. [24] who in a systematic review stated that though use of adjunctive drugs can provide statistically significant improvement but its clinical validity is questionable.
Microbiological parameter evaluated was Colony Forming Units (CFU) by anaerobic culturing. Anaerobic chamber with ‘Gas Pack’ was used in the present study as it provides strict anaerobiosis and it is convenient, easily available and economical. In Group A, CFU showed statistically significant reduction from baseline to 3 months (p<0.05). This might be attributed due to the antimicrobial activity of silver nanoparticles. Similar, antimicrobial activity of silver nanoparticles was observed by Kale S et al. [12] against Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans. Shao J et al. [25] also demonstrated antibacterial properties of silver nanoparticles in chitosan based membranes against Porphyromonas gingivalis and Fusobacterium nucleatum.
The significant reduction in group B is in accordance with previous in-vitro study done by Susanto C et al [26] who concluded that, the chitosan based Tetracycline gel is effective against A. actinomycetemcomitans, P. gingivalis and F. nucleatum. Similarly, Bosco JM et al [27] demonstrated antimicrobial activity of locally applied tetracycline solution. In Group C, also CFU was reduced significantly from baseline to 3 months (p<0.05). This can be due to reduction of bacterial load due to mechanical debridement.
In this era where the nanotechnology is gaining interest, silver nanoparticles can be used for non-surgical intervention to treat chronic periodontitis. This can be beneficial owing to the disadvantages of chemotherapeutic agents and increasing bacterial resistance. As compared to other therapeutic modalities employed against infection, silver nanoparticles gel is non-toxic at given concentration, has no side effects and does not require complicated application method or any complex armamentarium. It is well tolerated and well accepted by the patients. Silver nanoparticles gel with scaling and root planing gives promising results and it can definitely aid in periodontal diseases.














